Non-coding RNAs as drug targets
- PMID: 27444227
- PMCID: PMC5831170
- DOI: 10.1038/nrd.2016.117
Non-coding RNAs as drug targets
Abstract
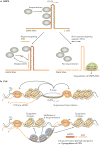
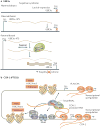
Most of the human genome encodes RNAs that do not code for proteins. These non-coding RNAs (ncRNAs) may affect normal gene expression and disease progression, making them a new class of targets for drug discovery. Because their mechanisms of action are often novel, developing drugs to target ncRNAs will involve equally novel challenges. However, many potential problems may already have been solved during the development of technologies to target mRNA. Here, we discuss the growing field of ncRNA - including microRNA, intronic RNA, repetitive RNA and long non-coding RNA - and assess the potential and challenges in their therapeutic exploitation.
Figures

References
-
- Ebbersen KK, Kjems J, Hansen TB. Circular RNAs: Identification, biogenesis and function. Biochim Biophys Acta. 2016;1859:163–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

