Dynamics of membrane nanotubes coated with I-BAR
- PMID: 27444356
- PMCID: PMC4956757
- DOI: 10.1038/srep30054
Dynamics of membrane nanotubes coated with I-BAR
Abstract
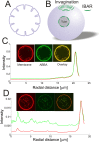
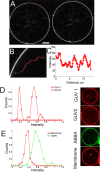
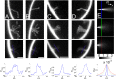
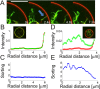
Membrane deformation is a necessary step in a number of cellular processes such as filopodia and invadopodia formation and has been shown to involve membrane shaping proteins containing membrane binding domains from the IRSp53-MIM protein family. In reconstituted membranes the membrane shaping domains can efficiently deform negatively charged membranes into tubules without any other proteins present. Here, we show that the IM domain (also called I-BAR domain) from the protein ABBA, forms semi-flexible nanotubes protruding into Giant Unilamellar lipid Vesicles (GUVs). By simultaneous quantification of tube intensity and tubular shape we find both the diameter and stiffness of the nanotubes. I-BAR decorated tubes were quantified to have a diameter of ~50 nm and exhibit no stiffening relative to protein free tubes of the same diameter. At high protein density the tubes are immobile whereas at lower density the tubes diffuse freely on the surface of the GUV. Bleaching experiments of the fluorescently tagged I-BAR confirmed that the mobility of the tubes correlates with the mobility of the I-BAR on the GUV membrane. Finally, at low density of I-BAR the protein upconcentrates within tubes protruding into the GUVs. This implies that I-BAR exhibits strong preference for negatively curved membranes.
Figures

References
-
- Doherty G. J. & McMahon H. T. Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 (2009). - PubMed
-
- Scholey J. M., Brust-Mascher I. & Mogilner A. Cell division. Nature 422, 746–752 (2003). - PubMed
-
- Leijsne N., Oddershede L. B. & Bendix P. M. An updated view at actin dynamics within filopodia. Cytoskeleton 72, 71–79 (2015). - PubMed
-
- Mattila P. K. & Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 9, 446–454 (2008). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

