Molecular mechanisms underlying alcohol-drinking behaviours
- PMID: 27444358
- PMCID: PMC5131788
- DOI: 10.1038/nrn.2016.85
Molecular mechanisms underlying alcohol-drinking behaviours
Abstract
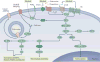
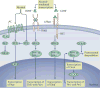
The main characteristic of alcohol use disorder is the consumption of large quantities of alcohol despite the negative consequences. The transition from the moderate use of alcohol to excessive, uncontrolled alcohol consumption results from neuroadaptations that cause aberrant motivational learning and memory processes. Here, we examine studies that have combined molecular and behavioural approaches in rodents to elucidate the molecular mechanisms that keep the social intake of alcohol in check, which we term 'stop pathways', and the neuroadaptations that underlie the transition from moderate to uncontrolled, excessive alcohol intake, which we term 'go pathways'. We also discuss post-transcriptional, genetic and epigenetic alterations that underlie both types of pathways.
Figures
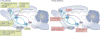
References
-
- World Health Organization. Global status report on alcohol and health 2014. WHO; 2014.
-
- Enoch MA, Goldman D. Problem drinking and alcoholism: diagnosis and treatment. Am Fam Physician. 2002;65:441–448. - PubMed
-
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders DSM-5. 5th. American Psychiatric Publishing; 2013.
-
- Koob GF. In: Behavioral Neurobiology of Alcohol Addiction. Sommer WH, Spanagel R, editors. Springer; 2013. pp. 3–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

