Role of Zn doping in oxidative stress mediated cytotoxicity of TiO2 nanoparticles in human breast cancer MCF-7 cells
- PMID: 27444578
- PMCID: PMC4957127
- DOI: 10.1038/srep30196
Role of Zn doping in oxidative stress mediated cytotoxicity of TiO2 nanoparticles in human breast cancer MCF-7 cells
Abstract
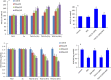
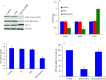
We investigated the effect of Zn-doping on structural and optical properties as well as cellular response of TiO2 nanoparticles (NPs) in human breast cancer MCF-7 cells. A library of Zn-doped (1-10 at wt%) TiO2 NPs was prepared. Characterization data indicated that dopant Zn was incorporated into the lattice of host TiO2. The average particle size of TiO2 NPs was decreases (38 to 28 nm) while the band gap energy was increases (3.35 eV-3.85 eV) with increasing the amount of Zn-doping. Cellular data demonstrated that Zn-doped TiO2 NPs induced cytotoxicity (cell viability reduction, membrane damage and cell cycle arrest) and oxidative stress (reactive oxygen species generation &glutathione depletion) in MCF-7 cells and toxic intensity was increases with increasing the concentration of Zn-doping. Molecular data revealed that Zn-doped TiO2 NPs induced the down-regulation of super oxide dismutase gene while the up-regulation of heme oxygenase-1 gene in MCF-7 cells. Cytotoxicity induced by Zn-doped TiO2 NPs was efficiently prevented by N-acetyl-cysteine suggesting that oxidative stress might be the primarily cause of toxicity. In conclusion, our data indicated that Zn-doping decreases the particle size and increases the band gap energy as well the oxidative stress-mediated toxicity of TiO2 NPs in MCF-7 cells.
Figures





References
-
- Nel A., Xia T., Madler L. & Li N. Toxic potential of materials at the nanolevel. Science 311, 622–627 (2006). - PubMed
-
- Jiang W., Kim B. S., Rutka J. T. & Chan W. W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3, 145–150 (2008). - PubMed
-
- Xia T. et al.. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 6, 1794–1807 (2006). - PubMed
-
- Setyawati M. I., Tay C. Y. & Leong D. T. Mechanistic investigation of the biological effects of SiO2, TiO2, and ZnO nanoparticles on intestinal cells. Small 11, 3458–3468 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

