Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure
- PMID: 27444755
- PMCID: PMC5378496
- DOI: 10.1161/CIRCRESAHA.116.309001
Proliferation and Recruitment Contribute to Myocardial Macrophage Expansion in Chronic Heart Failure
Abstract
Rationale: Macrophages reside in the healthy myocardium, participate in ischemic heart disease, and modulate myocardial infarction (MI) healing. Their origin and roles in post-MI remodeling of nonischemic remote myocardium, however, remain unclear.
Objective: This study investigated the number, origin, phenotype, and function of remote cardiac macrophages residing in the nonischemic myocardium in mice with chronic heart failure after coronary ligation.
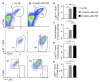
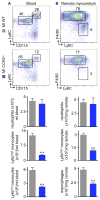
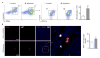
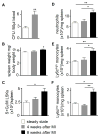
Methods and results: Eight weeks post MI, fate mapping and flow cytometry revealed that a 2.9-fold increase in remote macrophages results from both increased local macrophage proliferation and monocyte recruitment. Heart failure produced by extensive MI, through activation of the sympathetic nervous system, expanded medullary and extramedullary hematopoiesis. Circulating Ly6C(high) monocytes rose from 64±5 to 108±9 per microliter of blood (P<0.05). Cardiac monocyte recruitment declined in Ccr2(-/-) mice, reducing macrophage numbers in the failing myocardium. Mechanical strain of primary murine and human macrophage cultures promoted cell cycle entry, suggesting that the increased wall tension in post-MI heart failure stimulates local macrophage proliferation. Strained cells activated the mitogen-activated protein kinase pathway, whereas specific inhibitors of this pathway reduced macrophage proliferation in strained cell cultures and in the failing myocardium (P<0.05). Steady-state cardiac macrophages, monocyte-derived macrophages, and locally sourced macrophages isolated from failing myocardium expressed different genes in a pattern distinct from the M1/M2 macrophage polarization paradigm. In vivo silencing of endothelial cell adhesion molecules curbed post-MI monocyte recruitment to the remote myocardium and preserved ejection fraction (27.4±2.4 versus 19.1±2%; P<0.05).
Conclusions: Myocardial failure is influenced by an altered myeloid cell repertoire.
Keywords: heart failure; hypertrophy; macrophage; monocyte; myocardial infarction.
© 2016 American Heart Association, Inc.
Conflict of interest statement
J.E.D., and D.G.A. have filed intellectual property protection related to 7C1 nanoparticles. The authors declare that they have no further competing interests.
Figures



Comment in
-
Macrophages in the Remodeling Failing Heart.Circ Res. 2016 Sep 16;119(7):776-8. doi: 10.1161/CIRCRESAHA.116.309624. Circ Res. 2016. PMID: 27635078 Free PMC article. No abstract available.
References
-
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. - PubMed
-
- McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. - PubMed
-
- Landmesser U, Drexler H. Chronic heart failure: an overview of conventional treatment versus novel approaches. Nat Clin Pract Cardiovasc Med. 2005;2:628–638. - PubMed
-
- McMurray JJ. Clinical practice. Systolic heart failure. N Engl J Med. 2010;362:228–238. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

