Supratrigeminal Bilaterally Projecting Neurons Maintain Basal Tone and Enable Bilateral Phasic Activation of Jaw-Closing Muscles
- PMID: 27445144
- PMCID: PMC4951574
- DOI: 10.1523/JNEUROSCI.0839-16.2016
Supratrigeminal Bilaterally Projecting Neurons Maintain Basal Tone and Enable Bilateral Phasic Activation of Jaw-Closing Muscles
Abstract
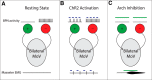
Anatomical studies have identified brainstem neurons that project bilaterally to left and right oromotor pools, which could potentially mediate bilateral muscle coordination. We use retrograde lentiviruses combined with a split-intein-mediated split-Cre-recombinase system in mice to isolate, characterize, and manipulate a population of neurons projecting to both the left and right jaw-closing trigeminal motoneurons. We find that these bilaterally projecting premotor neurons (BPNs) reside primarily in the supratrigeminal nucleus (SupV) and the parvicellular and intermediate reticular regions dorsal to the facial motor nucleus. These BPNs also project to multiple midbrain and brainstem targets implicated in orofacial sensorimotor control, and consist of a mix of glutamatergic, GABAergic, and glycinergic neurons, which can drive both excitatory and inhibitory inputs to trigeminal motoneurons when optogenetically activated in slice. Silencing BPNs with tetanus toxin light chain (TeNT) increases bilateral masseter activation during chewing, an effect driven by the expression of TeNT in SupV BPNs. Acute unilateral optogenetic inhibition of SupV BPNs identifies a group of tonically active neurons that function to lower masseter muscle tone, whereas unilateral optogenetic activation of SupV BPNs is sufficient to induce bilateral masseter activation both during resting state and during chewing. These results provide evidence for SupV BPNs in tonically modulating jaw-closing muscle tone and in mediating bilateral jaw closing.
Significance statement: We developed a method that combines retrograde lentiviruses with the split-intein-split-Cre system in mice to isolate, characterize, and manipulate neurons that project to both left and right jaw-closing motoneurons. We show that these bilaterally projecting premotor neurons (BPNs) reside primarily in the supratrigeminal nucleus and the rostral parvicellular and intermediate reticular nuclei. BPNs consist of both excitatory and inhibitory populations, and also project to multiple brainstem nuclei implicated in orofacial sensorimotor control. Manipulation of the supratrigeminal BPNs during natural jaw-closing behavior reveals a dual role for these neurons in eliciting phasic muscle activation and in maintaining basal muscle tone. The retrograde lentivirus carrying the split-intein-split-Cre system can be applied to study any neurons with bifurcating axons innervating two brain regions.
Keywords: bilaterally projecting neurons; optogenetics; retrograde lentivirus; split-intein-split-Cre system; supratrigeminal nucleus; trigeminal motor nucleus.
Copyright © 2016 the authors 0270-6474/16/367663-13$15.00/0.
Figures




References
-
- Balasubramaniam R, Ram S. Orofacial movement disorders. Oral Maxillofac Surg Clin North Am. 2008;20:273–285. vii. - PubMed
-
- Bourque MJ, Kolta A. Properties and interconnections of trigeminal interneurons of the lateral pontine reticular formation in the rat. J Neurophysiol. 2001;86:2583–2596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
