Dynamics of Propofol-Induced Loss of Consciousness Across Primate Neocortex
- PMID: 27445148
- PMCID: PMC4951576
- DOI: 10.1523/JNEUROSCI.4577-15.2016
Dynamics of Propofol-Induced Loss of Consciousness Across Primate Neocortex
Abstract
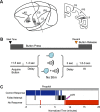
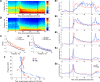
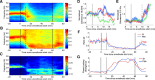
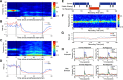
The precise neural mechanisms underlying transitions between consciousness and anesthetic-induced unconsciousness remain unclear. Here, we studied intracortical neuronal dynamics leading to propofol-induced unconsciousness by recording single-neuron activity and local field potentials directly in the functionally interconnecting somatosensory (S1) and frontal ventral premotor (PMv) network during a gradual behavioral transition from full alertness to loss of consciousness (LOC) and on through a deeper anesthetic level. Macaque monkeys were trained for a behavioral task designed to determine the trial-by-trial alertness and neuronal response to tactile and auditory stimulation. We show that disruption of coherent beta oscillations between S1 and PMv preceded, but did not coincide with, the LOC. LOC appeared to correspond to pronounced but brief gamma-/high-beta-band oscillations (lasting ∼3 min) in PMv, followed by a gamma peak in S1. We also demonstrate that the slow oscillations appeared after LOC in S1 and then in PMv after a delay, together suggesting that neuronal dynamics are very different across S1 versus PMv during LOC. Finally, neurons in both S1 and PMv transition from responding to bimodal (tactile and auditory) stimulation before LOC to only tactile modality during unconsciousness, consistent with an inhibition of multisensory integration in this network. Our results show that propofol-induced LOC is accompanied by spatiotemporally distinct oscillatory neuronal dynamics across the somatosensory and premotor network and suggest that a transitional state from wakefulness to unconsciousness is not a continuous process, but rather a series of discrete neural changes.
Significance statement: How information is processed by the brain during awake and anesthetized states and, crucially, during the transition is not clearly understood. We demonstrate that neuronal dynamics are very different within an interconnecting cortical network (primary somatosensory and frontal premotor area) during the loss of consciousness (LOC) induced by propofol in nonhuman primates. Coherent beta oscillations between these regions are disrupted before LOC. Pronounced but brief gamma-band oscillations appear to correspond to LOC. In addition, neurons in both of these cortices transition from responding to both tactile and auditory stimulation before LOC to only tactile modality during unconsciousness. We demonstrate that propofol-induced LOC is accompanied by spatiotemporally distinctive neuronal dynamics in this network with concurrent changes in multisensory processing.
Keywords: general anesthesia; local field potential; loss of consciousness; primate; sensory premotor network; single-neuron activity.
Copyright © 2016 the authors 0270-6474/16/367718-09$15.00/0.
Figures

References
-
- Akeju O, Pavone KJ, Westover MB, Vazquez R, Prerau MJ, Harrell PG, Hartnack KE, Rhee J, Sampson AL, Habeeb K, Gao L, Pierce ET, Walsh JL, Brown EN, Purdon PL. A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology. 2014;121:978–989. doi: 10.1097/ALN.0000000000000419. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
