The Severity of Infection Determines the Localization of Damage and Extent of Sensorineural Hearing Loss in Experimental Pneumococcal Meningitis
- PMID: 27445150
- PMCID: PMC6705551
- DOI: 10.1523/JNEUROSCI.0554-16.2016
The Severity of Infection Determines the Localization of Damage and Extent of Sensorineural Hearing Loss in Experimental Pneumococcal Meningitis
Abstract
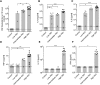
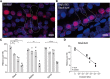
Hearing loss is an important sequela of pneumococcal meningitis (PM), occurring in up to 30% of survivors. The role of the severity of infection on hearing function and pathomorphological consequences in the cochlea secondary to PM have not been investigated to date. Using a well-established model of PM, we systematically investigated the functional hearing outcome and the long-term fate of neurosensory cells in the cochlea, i.e., hair cells and spiral ganglion neurons (SGNs), with a focus on their tonotopic distribution. Intracisternal infection of infant rats with increasing inocula of Streptococcus pneumoniae resulted in a dose-dependent increase in CSF levels of interleukin-1β, interleukin-6, tumor necrosis factor α, interleukin-10, and interferon-γ in acute disease. The severity of long-term hearing loss at 3 weeks after infection, measured by auditory brainstem response recordings, correlated to the initial inoculum dose and to the levels of proinflammatory cytokines determined in the acute phase of PM. Quantitative cochlear histomorphology revealed a significant loss of SGNs and outer hair cells that strongly correlated to the level of infection, with the most severe damage occurring in the basal part of the cochlea. Inner hair cells (IHCs) were not significantly affected throughout the entire cochlea. However, surviving IHCs lost synaptic connectivity to remaining SGNs in all cochlear regions. These findings provide evidence that the inoculum concentration, i.e., severity of infection, is the major determinant of long-term morphological cell pathologies in the cochlea and functional hearing loss.
Significance statement: Hearing loss is a neurofunctional deficit occurring in up to 30% of patients surviving pneumococcal meningitis (PM). Here, we analyze the correlation between the severity of infection and the inflammatory response in the CSF, the tonotopic distribution of neurosensory pathologies in the cochlea, and the long-term hearing function in a rat model of pneumococcal meningitis. Our study identifies the severity of infection as the key determinant of long-term hearing loss, underlining the importance of the prompt institution of antibiotic therapy in patients suffering from PM. Furthermore, our findings reveal in detail the spatial loss of cochlear neurosensory cells, providing new insights into the pathogenesis of meningitis-associated hearing loss that reveal new starting points for the development of otoprotective therapies.
Keywords: animal model; hair cells; pneumococcal meningitis; sensorineural hearing loss; spiral ganglion neurons; streptococcus pneumonia.
Copyright © 2016 the authors 0270-6474/16/367740-10$15.00/0.
Figures


References
-
- Brand Y, Senn P, Kompis M, Dillier N, Allum JH. Cochlear implantation in children and adults in Switzerland. Swiss Med Wkly. 2014;144:w13909. - PubMed
-
- Brandt CT, Cayé-Thomasen P, Lund SP, Worsøe L, Ostergaard C, Frimodt-Møller N, Espersen F, Thomsen J, Lundgren JD. Hearing loss and cochlear damage in experimental pneumococcal meningitis, with special reference to the role of neutrophil granulocytes. Neurobiol Dis. 2006;23:300–311. doi: 10.1016/j.nbd.2006.03.006. - DOI - PubMed
-
- Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24:733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
