Chemically Programmed Bispecific Antibodies in Diabody Format
- PMID: 27445334
- PMCID: PMC5016699
- DOI: 10.1074/jbc.M116.745588
Chemically Programmed Bispecific Antibodies in Diabody Format
Abstract
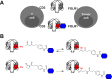
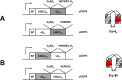
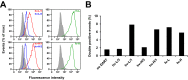
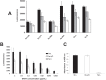
Chemically programmed bispecific antibodies (biAbs) endow target cell-binding small molecules with the ability to recruit and activate effector cells of the immune system. Here we report a platform of chemically programmed biAbs aimed at redirecting cytotoxic T cells to eliminate cancer cells. Two different antibody technologies were merged together to make a novel chemically programmed biAb. This was achieved by combining the humanized anti-hapten monoclonal antibody (mAb) h38C2 with the humanized anti-human CD3 mAb v9 in a clinically investigated diabody format known as Dual-Affinity Re-Targeting (DART). We show that h38C2 × v9 DARTs can readily be equipped with tumor-targeting hapten-derivatized small molecules without causing a systemic response harming healthy tissues. As a proof of concept, we chemically programmed h38C2 × v9 with hapten-folate and demonstrated its selectivity and potency against folate receptor 1 (FOLR1)-expressing ovarian cancer cells in vitro and in vivo Unlike conventional biAbs, chemically programmed biAbs in DART format are highly modular with broad utility in terms of both target and effector cell engagement. Most importantly, they provide tumor-targeting compounds access to the power of cancer immunotherapy.
Keywords: T-cell; antibody engineering; cancer therapy; chemical modification; folate; immunotherapy; ovarian cancer.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Kontermann R. E., and Brinkmann U. (2015) Bispecific antibodies. Drug Discov. Today 20, 838–847 - PubMed
-
- Frankel S. R., and Baeuerle P. A. (2013) Targeting T cells to tumor cells using bispecific antibodies. Curr. Opin. Chem. Biol. 17, 385–392 - PubMed
-
- Perez P., Hoffman R. W., Shaw S., Bluestone J. A., and Segal D. M. (1985) Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature 316, 354–356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

