Therapeutics Insight with Inclusive Immunopharmacology Explication of Human Rotavirus A for the Treatment of Diarrhea
- PMID: 27445802
- PMCID: PMC4917548
- DOI: 10.3389/fphar.2016.00153
Therapeutics Insight with Inclusive Immunopharmacology Explication of Human Rotavirus A for the Treatment of Diarrhea
Abstract
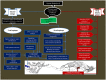
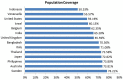
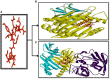
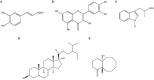
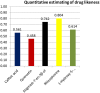
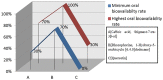
Rotavirus is the most common cause of severe infant and childhood diarrhea worldwide, and the morbidity and mortality rate is going to be outnumbered in developing countries like Bangladesh. To mitigate this substantial burden of disease, new therapeutics such as vaccine and drug are swiftly required against rotavirus. The present therapeutics insight study was performed with comprehensive immunoinformatics and pharmacoinformatics approach. T and B-cell epitopes were assessed in the conserved region of outer capsid protein VP4 among the highly reviewed strains from different countries including Bangladesh. The results suggest that epitope SU1 (TLKNLNDNY) could be an ideal candidate among the predicted five epitopes for both T and B-cell epitopes for the development of universal vaccine against rotavirus. This research also suggests five novel drug compounds from medicinal plant Rhizophora mucronata Lamk. for better therapeutics strategies against rotavirus diarrhea based on 3D structure building, pharmacophore, ADMET, and QSAR properties. The exact mode of action between drug compounds and target protein VP4 were revealed by molecular docking analysis. Drug likeness and oral bioavailability further confirmed the effectiveness of the proposed drugs against rotavirus diarrhea. This study might be implemented for experimental validation to facilitate the novel vaccine and drug design.
Keywords: diarrhea; drug development; human rotavirus; immunoinformatics; pharmacoinformatics; vaccine design.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

