Bradykinin Induces TRPV1 Exocytotic Recruitment in Peptidergic Nociceptors
- PMID: 27445816
- PMCID: PMC4917537
- DOI: 10.3389/fphar.2016.00178
Bradykinin Induces TRPV1 Exocytotic Recruitment in Peptidergic Nociceptors
Abstract
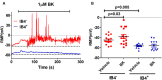
Transient receptor potential vanilloid I (TRPV1) sensitization in peripheral nociceptors is a prominent phenomenon that occurs in inflammatory pain conditions. Pro-algesic agents can potentiate TRPV1 activity in nociceptors through both stimulation of its channel gating and mobilization of channels to the neuronal surface in a context dependent manner. A recent study reported that ATP-induced TRPV1 sensitization in peptidergic nociceptors involves the exocytotic release of channels trafficked by large dense core vesicles (LDCVs) that cargo alpha-calcitonin gene related peptide alpha (αCGRP). We hypothesized that, similar to ATP, bradykinin may also use different mechanisms to sensitize TRPV1 channels in peptidergic and non-peptidergic nociceptors. We found that bradykinin notably enhances the excitability of peptidergic nociceptors, and sensitizes TRPV1, primarily through the bradykinin receptor 2 pathway. Notably, bradykinin sensitization of TRPV1 in peptidergic nociceptors was significantly blocked by inhibiting Ca(2+)-dependent neuronal exocytosis. In addition, silencing αCGRP gene expression, but not substance P, drastically reduced bradykinin-induced TRPV1 sensitization in peptidergic nociceptors. Taken together, these findings indicate that bradykinin-induced sensitization of TRPV1 in peptidergic nociceptors is partially mediated by the exocytotic mobilization of new channels trafficked by αCGRP-loaded LDCVs to the neuronal membrane. Our findings further imply a central role of αCGRP peptidergic nociceptors in peripheral algesic sensitization, and substantiate that inhibition of LDCVs exocytosis is a valuable therapeutic strategy to treat pain, as it concurrently reduces the release of pro-inflammatory peptides and the membrane recruitment of thermoTRP channels.
Keywords: TRPV1; exocytosis; inflammation; neuropeptides; nociceptors.
Figures







References
-
- Baker M. D., Bostock H. (1997). Low-threshold, persistent sodium current in rat large dorsal root ganglion neurons in culture. J. Neurophysiol. 77 1503–1513. - PubMed
-
- Cavanaugh D. J., Chesler A. T., Braz J. M., Shah N. M., Julius D., Basbaum A. I. (2011). Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J. Neurosci. 31 10119–10127. 10.1523/JNEUROSCI.1299-11.2011 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

