Uptake of Plasmin-PN-1 Complexes in Early Human Atheroma
- PMID: 27445860
- PMCID: PMC4927630
- DOI: 10.3389/fphys.2016.00273
Uptake of Plasmin-PN-1 Complexes in Early Human Atheroma
Abstract
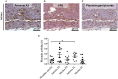
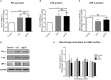
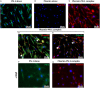
Zymogens are delivered to the arterial wall by radial transmural convection. Plasminogen can be activated within the arterial wall to produce plasmin, which is involved in evolution of the atherosclerotic plaque. Vascular smooth muscle cells (vSMCs) protect the vessels from proteolytic injury due to atherosclerosis development by highly expressing endocytic LDL receptor-related protein-1 (LRP-1), and by producing anti-proteases, such as Protease Nexin-1 (PN-1). PN-1 is able to form covalent complexes with plasmin. We hypothesized that plasmin-PN-1 complexes could be internalized via LRP-1 by vSMCs during the early stages of human atheroma. LRP-1 is also responsible for the capture of aggregated LDL in human atheroma. Plasmin activity and immunohistochemical analyses of early human atheroma showed that the plasminergic system is activated within the arterial wall, where intimal foam cells, including vSMCs and platelets, are the major sites of PN-1 accumulation. Both PN-1 and LRP-1 are overexpressed in early atheroma at both messenger and protein levels. Cell biology studies demonstrated an increased expression of PN-1 and tissue plasminogen activator by vSMCs in response to LDL. Plasmin-PN-1 complexes are internalized via LRP-1 in vSMCs, whereas plasmin alone is not. Tissue PN-1 interacts with plasmin in early human atheroma via two complementary mechanisms: plasmin inhibition and tissue uptake of plasmin-PN-1 complexes via LRP-1 in vSMCs. Despite this potential protective effect, plasminogen activation by vSMCs remains abnormally elevated in the intima in early stages of human atheroma.
Keywords: and atherosclerosis; antiproteases; endocytosis; proteases; vascular smooth muscle cells.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

