Bone Marrow Adipose Tissue: To Be or Not To Be a Typical Adipose Tissue?
- PMID: 27445987
- PMCID: PMC4928601
- DOI: 10.3389/fendo.2016.00085
Bone Marrow Adipose Tissue: To Be or Not To Be a Typical Adipose Tissue?
Abstract
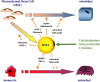
Bone marrow adipose tissue (BMAT) emerges as a distinct fat depot whose importance has been proved in the bone-fat interaction. Indeed, it is well recognized that adipokines and free fatty acids released by adipocytes can directly or indirectly interfere with cells of bone remodeling or hematopoiesis. In pathological states, such as osteoporosis, each of adipose tissues - subcutaneous white adipose tissue (WAT), visceral WAT, brown adipose tissue (BAT), and BMAT - is differently associated with bone mineral density (BMD) variations. However, compared with the other fat depots, BMAT displays striking features that makes it a substantial actor in bone alterations. BMAT quantity is well associated with BMD loss in aging, menopause, and other metabolic conditions, such as anorexia nervosa. Consequently, BMAT is sensed as a relevant marker of a compromised bone integrity. However, analyses of BMAT development in metabolic diseases (obesity and diabetes) are scarce and should be, thus, more systematically addressed to better apprehend the bone modifications in that pathophysiological contexts. Moreover, bone marrow (BM) adipogenesis occurs throughout the whole life at different rates. Following an ordered spatiotemporal expansion, BMAT has turned to be a heterogeneous fat depot whose adipocytes diverge in their phenotype and their response to stimuli according to their location in bone and BM. In vitro, in vivo, and clinical studies point to a detrimental role of BM adipocytes (BMAs) throughout the release of paracrine factors that modulate osteoblast and/or osteoclast formation and function. However, the anatomical dissemination and the difficulties to access BMAs still hamper our understanding of the relative contribution of BMAT secretions compared with those of peripheral adipose tissues. A further characterization of the phenotype and the functional regulation of BMAs are ever more required. Based on currently available data and comparison with other fat tissues, this review addresses the originality of the BMAT with regard to its development, anatomy, metabolic properties, and response to physiological cues.
Keywords: adipokine; bone fragility; bone marrow adiposity; fat–bone association; marrow fat; osteoporosis; skeletal adipocyte.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

