Development of a Phage Cocktail to Control Proteus mirabilis Catheter-associated Urinary Tract Infections
- PMID: 27446059
- PMCID: PMC4923195
- DOI: 10.3389/fmicb.2016.01024
Development of a Phage Cocktail to Control Proteus mirabilis Catheter-associated Urinary Tract Infections
Abstract
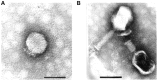
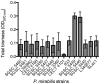
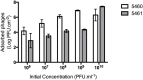
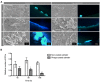
Proteus mirabilis is an enterobacterium that causes catheter-associated urinary tract infections (CAUTIs) due to its ability to colonize and form crystalline biofilms on the catheters surface. CAUTIs are very difficult to treat, since biofilm structures are highly tolerant to antibiotics. Phages have been used widely to control a diversity of bacterial species, however, a limited number of phages for P. mirabilis have been isolated and studied. Here we report the isolation of two novel virulent phages, the podovirus vB_PmiP_5460 and the myovirus vB_PmiM_5461, which are able to target, respectively, 16 of the 26 and all the Proteus strains tested in this study. Both phages have been characterized thoroughly and sequencing data revealed no traces of genes associated with lysogeny. To further evaluate the phages' ability to prevent catheter's colonization by Proteus, the phages adherence to silicone surfaces was assessed. Further tests in phage-coated catheters using a dynamic biofilm model simulating CAUTIs, have shown a significant reduction of P. mirabilis biofilm formation up to 168 h of catheterization. These results highlight the potential usefulness of the two isolated phages for the prevention of surface colonization by this bacterium.
Keywords: Proteus mirabilis; bacteriophage therapy; bacteriophages; biofilms; phage cocktail; urinary tract infection.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

