Use of a biological reactor and platelet-rich plasma for the construction of tissue-engineered bone to repair articular cartilage defects
- PMID: 27446265
- PMCID: PMC4950899
- DOI: 10.3892/etm.2016.3380
Use of a biological reactor and platelet-rich plasma for the construction of tissue-engineered bone to repair articular cartilage defects
Abstract
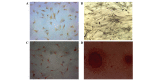
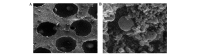
Articular cartilage defects are a major clinical burden worldwide. Current methods to repair bone defects include bone autografts, allografts and external fixation. In recent years, the repair of bone defects by tissue engineering has emerged as a promising approach. The present study aimed to assess a novel method using a biological reactor with platelet-rich plasma to construct tissue-engineered bone. Beagle bone marrow mesenchymal stem cells (BMSCs) were isolated and differentiated into osteoblasts and chondroblasts using platelet-rich plasma and tricalcium phosphate scaffolds cultured in a bioreactor for 3 weeks. The cell scaffold composites were examined by scanning electron microscopy (SEM) and implanted into beagles with articular cartilage defects. The expression of osteogenic markers, alkaline phosphatase and bone γ-carboxyglutamate protein (BGLAP) were assessed using polymerase chain reaction after 3 months. Articular cartilage specimens were observed histologically. Adhesion and distribution of BMSCs on the β-tricalcium phosphate (β-TCP) scaffold were confirmed by SEM. Histological examination revealed that in vivo bone defects were largely repaired 12 weeks following implantation. The expression levels of alkaline phosphatase (ALP) and BGLAP in the experimental groups were significantly elevated compared with the negative controls. BMSCs may be optimum seed cells for tissue engineering in bone repair. Platelet-rich plasma (PRP) provides a rich source of cytokines to promote BMSC function. The β-TCP scaffold is advantageous for tissue engineering due to its biocompatibility and 3D structure that promotes cell adhesion, growth and differentiation. The tissue-engineered bone was constructed in a bioreactor using BMSCs, β-TCP scaffolds and PRP and displayed appropriate morphology and biological function. The present study provides an efficient method for the generation of tissue-engineered bone for cartilage repair, compared with previously used methods.
Keywords: bioreactor; bone regeneration; bone repair; bone tissue engineering; platelet-rich plasma.
Figures





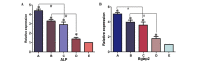
Similar articles
-
Quantitative analysis of factors influencing tissue-engineered bone formation by detecting the expression levels of alkaline phosphatase and bone γ-carboxyglutamate protein 2.Exp Ther Med. 2015 Apr;9(4):1097-1102. doi: 10.3892/etm.2015.2259. Epub 2015 Feb 4. Exp Ther Med. 2015. PMID: 25780393 Free PMC article.
-
Construction of tissue-engineered bone using a bioreactor and platelet-rich plasma.Exp Ther Med. 2014 Aug;8(2):413-418. doi: 10.3892/etm.2014.1774. Epub 2014 Jun 11. Exp Ther Med. 2014. PMID: 25009593 Free PMC article.
-
In vivo ossification of a scaffold combining β-tricalcium phosphate and platelet-rich plasma.Exp Ther Med. 2014 Nov;8(5):1381-1388. doi: 10.3892/etm.2014.1969. Epub 2014 Sep 15. Exp Ther Med. 2014. PMID: 25289027 Free PMC article.
-
Bone Marrow Stem Cells with Tissue-Engineered Scaffolds for Large Bone Segmental Defects: A Systematic Review.Tissue Eng Part B Rev. 2023 Oct;29(5):457-472. doi: 10.1089/ten.TEB.2022.0213. Epub 2023 Apr 20. Tissue Eng Part B Rev. 2023. PMID: 36905366 Review.
-
Progress of Platelet Derivatives for Cartilage Tissue Engineering.Front Bioeng Biotechnol. 2022 Jun 16;10:907356. doi: 10.3389/fbioe.2022.907356. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35782516 Free PMC article. Review.
Cited by
-
Recent Advances in Tissue Engineering Strategies for the Treatment of Joint Damage.Curr Rheumatol Rep. 2017 Aug;19(8):44. doi: 10.1007/s11926-017-0671-7. Curr Rheumatol Rep. 2017. PMID: 28718059 Review.
-
Platelet-rich plasma inhibits RANKL-induced osteoclast differentiation through activation of Wnt pathway during bone remodeling.Int J Mol Med. 2018 Feb;41(2):729-738. doi: 10.3892/ijmm.2017.3258. Epub 2017 Nov 16. Int J Mol Med. 2018. PMID: 29207140 Free PMC article.
-
Tissue engineering strategies hold promise for the repair of articular cartilage injury.Biomed Eng Online. 2024 Sep 11;23(1):92. doi: 10.1186/s12938-024-01260-w. Biomed Eng Online. 2024. PMID: 39261876 Free PMC article. Review.
-
Management of Hepple Stage V Osteochondral Lesion of the Talus with a Platelet-Rich Plasma Scaffold.Biomed Res Int. 2017;2017:6525373. doi: 10.1155/2017/6525373. Epub 2017 Mar 16. Biomed Res Int. 2017. PMID: 28401159 Free PMC article.
-
Bone engineering by cell sheet technology to repair mandibular defects.Exp Ther Med. 2017 Nov;14(5):5007-5011. doi: 10.3892/etm.2017.5118. Epub 2017 Sep 15. Exp Ther Med. 2017. PMID: 29201205 Free PMC article.
References
-
- Vacanti CA, Upton J. Tissue-engineered morphogenesis of cartilage and bone by means of cell transplantation using synthetic biodegradable polymer matrices. Clin Plast Surg. 1994;21:445–62. - PubMed
-
- Zou D, Zhang Z, Ye D, Tang A, Deng L, Han W, Zhao J, Wang S, Zhang W, Zhu C, et al. Repair of critical-sized rat calvarial defects using genetically engineered bone marrow-derived mesenchymal stem cells overexpressing hypoxia-inducible factor-1α. Stem Cells. 2011;29:1380–1390. - PubMed
-
- Sun S, Ren Q, Wang D, Zhang L, Wu S, Sun XT. Repairing cartilage defects using chondrocyte and osteoblast composites developed using a bioreactor. Chin Med J (Engl) 2011;124:758–763. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
