Label-retaining assay enriches tumor-initiating cells in glioblastoma spheres cultivated in serum-free medium
- PMID: 27446356
- PMCID: PMC4950123
- DOI: 10.3892/ol.2016.4690
Label-retaining assay enriches tumor-initiating cells in glioblastoma spheres cultivated in serum-free medium
Abstract
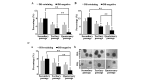
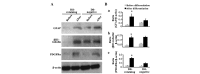
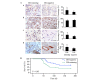
Label-retaining cells, which are characterized by dormancy or slow cycling, may be identified in a number of human normal and cancer tissues, and these cells demonstrate stem cell potential. In glioblastoma, label-retaining assays to enrich glioma stem cells remain to be fully investigated. In the present study, glioblastoma sphere cells cultured in serum-free medium were initially stained with the cell membrane fluorescent marker DiI. The fluorescence intensity during cell proliferation and sphere reformation was observed. At 2 weeks, the DiI-retaining cells were screened by fluorescence-activated cell sorting and compared phenotypically with the DiI-negative cells in terms of in vitro proliferation, clonogenicity and multipotency and for in vivo tumorigenicity, as well as sensitivity to irradiation and temozolomide treatment. It was observed that DiI-retaining cells accounted for a small proportion, <10%, within the glioblastoma spheres and that DiI-retaining cells proliferated significantly more slowly compared with DiI-negative cells (P=0.011, P=0.035 and P=0.023 in the of NCH421k, NCH441 and NCH644 glioblastoma sphere cell lines). Significantly increased clonogenicity (P=0.002, P=0.034 and P=0.016 in the NCH441, NCH644 and NCH421k glioblastoma sphere cell lines) and three-lineage multipotency were observed in DiI-retaining cells in vitro compared with DiI-negative cells. As few as 100 DiI-retaining cells were able to effectively generate tumors in the immunocompromised mouse brain, whereas the same number of DiI-negative cells possessed no such ability, indicating the increased tumorigenicity of DiI-retaining cells compared with DiI-negative cells. Furthermore, DiI-retaining cells demonstrated significant resistance following irradiation (P=0.012, P=0.024 and P=0.036) and temozolomide (P=0.003, P=0.005 and P=0.029) compared with DiI-negative cells in the NCH421k, NCH441 and NCH644 glioblastoma sphere cell lines, respectively. It was concluded that label-retaining cells in glioblastoma spheres manifest clear stem cell features and that the label-retaining assay may be utilized to further enrich glioma stem cells cultured under serum-free conditions for additional study.
Keywords: DiI; cancer stem cell; glioma; label-retaining assay; label-retaining cell.
Figures


References
-
- Rock K, McArdle O, Forde P, Dunne M, Fitzpatrick D, O'Neill B, Faul C. A clinical review of treatment outcomes in glioblastoma multiforme - the validation in a non-trial population of the results of a randomised Phase III clinical trial: Has a more radical approach improved survival? Br J Radiol. 2012;85:e729–e733. doi: 10.1259/bjr/83796755. - DOI - PMC - PubMed
-
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
