SIX1 coordinates with TGFβ signals to induce epithelial-mesenchymal transition in cervical cancer
- PMID: 27446426
- PMCID: PMC4950046
- DOI: 10.3892/ol.2016.4797
SIX1 coordinates with TGFβ signals to induce epithelial-mesenchymal transition in cervical cancer
Abstract
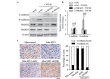
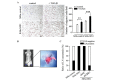
Epithelial-mesenchymal transition (EMT) plays a critical role in promoting tumor invasion and metastasis. However, the key cofactors that modulate the signal transduction to induce EMT have note been fully explored to date. The present study reports that sine oculis homeobox homolog 1 (SIX1) is able to promote EMT of cervical cancer by coordinating with transforming growth factor (TGF)β-SMAD signals. The expression of SIX1 was negatively correlated with the expression of the epithelial marker E-cadherin in two independent groups of cervical cancer specimens. SIX1 could promote the transition of mesenchymal phenotype in the presence of active TGFβ signals in vitro and in vivo. TGFβ-SMAD signals were required for the SIX1-mediated promotion of EMT and metastatic capacity of cervical cancer cells. Together, SIX1 and TGFβ cooperated to induce more remarkable changes in the transition of phenotype than each of them alone, and coordinated to promote cell motility and tumor metastasis in cervical cancer. These results suggest that the coordination of SIX1 and TGFβ signals may be crucial in the EMT program, and that SIX1/TGFβ may be considered a valuable marker for evaluating the metastatic potential of cervical cancer cells, or a therapeutic target in the treatment of cervical cancer.
Keywords: EMT; SIX1; TGFβ; cervical cancer; metastasis.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
