Catalytic Promiscuity of the Radical S-adenosyl-L-methionine Enzyme NosL
- PMID: 27446906
- PMCID: PMC4916742
- DOI: 10.3389/fchem.2016.00027
Catalytic Promiscuity of the Radical S-adenosyl-L-methionine Enzyme NosL
Abstract
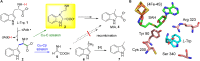
Catalytic promiscuity plays a key role in enzyme evolution and the acquisition of novel biological functions. Because of the high reactivity of radical species, in our view enzymes involving radical-mediated mechanisms could intrinsically be more prone to catalytic promiscuity. This mini-review summarizes the recent advances in the study of NosL, a radical S-adenosyl-L-methionine (SAM)-dependent L-tryptophan (L-Trp) lyase. We demonstrate here the interesting chemistry and remarkable catalytic promiscuity of NosL, and attempt to highlight the high evolvability of radical SAM enzymes and the potential to engineer these enzymes for novel and improved activities.
Keywords: biosynthesis; enzyme engineering; evolution; metalloenzyme; promiscuity.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

