Intestinal dysbiosis contributes to the delayed gastrointestinal transit in high-fat diet fed mice
- PMID: 27446985
- PMCID: PMC4945127
- DOI: 10.1016/j.jcmgh.2015.12.008
Intestinal dysbiosis contributes to the delayed gastrointestinal transit in high-fat diet fed mice
Abstract
Background & aims: High-fat diet (HFD) feeding is associated with gastrointestinal motility disorders. We recently reported delayed colonic motility in mice fed a HFD mice for 11 weeks. In this study, we investigated the contributing role of gut microbiota in HFD-induced gut dysmotility.
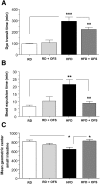
Methods: Male C57BL/6 mice were fed a HFD (60% kcal fat) or a regular/control diet (RD) (18% kcal fat) for 13 weeks. Serum and fecal endotoxin levels were measured, and relative amounts of specific gut bacteria in the feces assessed by real time PCR. Intestinal transit was measured by fluorescent-labeled marker and bead expulsion test. Enteric neurons were assessed by immunostaining. Oligofructose (OFS) supplementation with RD or HFD for 5 weeks was also studied. In vitro studies were performed using primary enteric neurons and an enteric neuronal cell line.
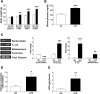
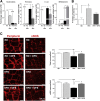
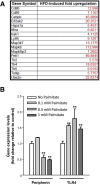
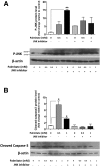
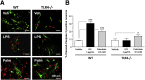
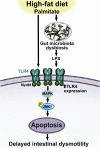
Results: HFD-fed mice had reduced numbers of enteric nitrergic neurons and exhibited delayed gastrointestinal transit compared to RD-fed mice. HFD-fed mice had higher fecal Firmicutes and Escherichia coli and lower Bacteroidetes compared to RD-fed mice. OFS supplementation protected against enteric nitrergic neurons loss in HFD-fed mice, and improved intestinal transit time. OFS supplementation resulted in a reductions in fecal Firmicutes and Escherichia coli and serum endotoxin levels. In vitro, palmitate activation of TLR4 induced enteric neuronal apoptosis in a p-JNK1 dependent pathway. This apoptosis was prevented by a JNK inhibitor and in neurons from TLR4-/- mice.
Conclusions: Together our data suggest that intestinal dysbiosis in HFD fed mice contribute to the delayed intestinal motility by inducing a TLR4-dependant neuronal loss. Manipulation of gut microbiota with OFS improved intestinal motility in HFD mice.
Keywords: LPS; Myenteric neurons; TLR4; colon transit; gut microbiota; palmitate.
Figures
References
-
- Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manag Care Interface. 2006;19:43–49. - PubMed
-
- Bharucha A.E., Dorn S.D., Lembo A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144:211–217. - PubMed
-
- vd Baan-Slootweg O.H., Liem O., Bekkali N. Constipation and colonic transit times in children with morbid obesity. J Pediatr Gastroenterol Nutr. 2011;52:442–445. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
