ASPP2 deficiency causes features of 1q41q42 microdeletion syndrome
- PMID: 27447114
- PMCID: PMC5136487
- DOI: 10.1038/cdd.2016.76
ASPP2 deficiency causes features of 1q41q42 microdeletion syndrome
Abstract
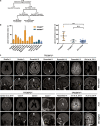
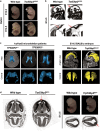
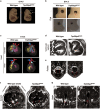
Chromosomal abnormalities are implicated in a substantial number of human developmental syndromes, but for many such disorders little is known about the causative genes. The recently described 1q41q42 microdeletion syndrome is characterized by characteristic dysmorphic features, intellectual disability and brain morphological abnormalities, but the precise genetic basis for these abnormalities remains unknown. Here, our detailed analysis of the genetic abnormalities of 1q41q42 microdeletion cases identified TP53BP2, which encodes apoptosis-stimulating protein of p53 2 (ASPP2), as a candidate gene for brain abnormalities. Consistent with this, Trp53bp2-deficient mice show dilation of lateral ventricles resembling the phenotype of 1q41q42 microdeletion patients. Trp53bp2 deficiency causes 100% neonatal lethality in the C57BL/6 background associated with a high incidence of neural tube defects and a range of developmental abnormalities such as congenital heart defects, coloboma, microphthalmia, urogenital and craniofacial abnormalities. Interestingly, abnormalities show a high degree of overlap with 1q41q42 microdeletion-associated abnormalities. These findings identify TP53BP2 as a strong candidate causative gene for central nervous system (CNS) defects in 1q41q42 microdeletion syndrome, and open new avenues for investigation of the mechanisms underlying CNS abnormalities.
Figures


Similar articles
-
The discovery of microdeletion syndromes in the post-genomic era: review of the methodology and characterization of a new 1q41q42 microdeletion syndrome.Genet Med. 2007 Sep;9(9):607-16. doi: 10.1097/gim.0b013e3181484b49. Genet Med. 2007. PMID: 17873649 Review.
-
Phenotypic features of 1q41q42 microdeletion including WDR26 and FBXO28 are clinically recognizable: The first case from Japan.Brain Dev. 2019 May;41(5):452-455. doi: 10.1016/j.braindev.2018.12.006. Epub 2019 Jan 8. Brain Dev. 2019. PMID: 30635136
-
Refinement of the critical region of 1q41q42 microdeletion syndrome identifies FBXO28 as a candidate causative gene for intellectual disability and seizures.Am J Med Genet A. 2014 Feb;164A(2):441-8. doi: 10.1002/ajmg.a.36320. Epub 2013 Dec 19. Am J Med Genet A. 2014. PMID: 24357076 Review.
-
New cases and refinement of the critical region in the 1q41q42 microdeletion syndrome.Eur J Med Genet. 2011 Jan-Feb;54(1):42-9. doi: 10.1016/j.ejmg.2010.10.002. Epub 2010 Oct 15. Eur J Med Genet. 2011. PMID: 20951845
-
Clinical characterization of DISP1 haploinsufficiency: A case report.Eur J Med Genet. 2013 Jun;56(6):309-13. doi: 10.1016/j.ejmg.2013.03.007. Epub 2013 Mar 28. Eur J Med Genet. 2013. PMID: 23542665
Cited by
-
ASPPs multimerize protein phosphatase 1.bioRxiv [Preprint]. 2025 May 19:2025.05.16.654433. doi: 10.1101/2025.05.16.654433. bioRxiv. 2025. PMID: 40475493 Free PMC article. Preprint.
-
Quantitative Image Processing for Three-Dimensional Episcopic Images of Biological Structures: Current State and Future Directions.Biomedicines. 2023 Mar 15;11(3):909. doi: 10.3390/biomedicines11030909. Biomedicines. 2023. PMID: 36979887 Free PMC article. Review.
-
The Complement Regulator Susd4 Influences Nervous-System Function and Neuronal Morphology in Mice.iScience. 2020 Mar 27;23(3):100957. doi: 10.1016/j.isci.2020.100957. Epub 2020 Feb 28. iScience. 2020. PMID: 32179479 Free PMC article.
References
-
- Tyshchenko N, Lurie I, Schinzel A. Chromosomal map of human brain malformations. Hum Genet 2008; 124: 73–80. - PubMed
-
- Rosenfeld JA, Lacassie Y, El-Khechen D, Escobar LF, Reggin J, Heuer C et al. New cases and refinement of the critical region in the 1q41q42 microdeletion syndrome. Eur J Med Genet 2011; 54: 42–49. - PubMed
-
- Au PYB, Argiropoulos B, Parboosingh JS, Micheil Innes A. Refinement of the critical region of 1q41q42 microdeletion syndrome identifies FBXO28 as a candidate causative gene for intellectual disability and seizures. Am J Med Genet A 2014; 164: 441–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

