Direct detection of nitrotyrosine-containing proteins using an aniline-based oxidative coupling strategy
- PMID: 27447346
- PMCID: PMC5001876
- DOI: 10.1039/c6cc04575h
Direct detection of nitrotyrosine-containing proteins using an aniline-based oxidative coupling strategy
Abstract
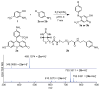
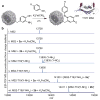
A convenient two-step method is described for the detection of nitrotyrosine-containing proteins. First, nitrotyrosines are reduced to aminophenols using sodium dithionite. Following this, an oxidative coupling reaction is used to attach anilines bearing fluorescence reporters or affinity probes. Features of this approach include fast reaction times, pmol-level sensitivity, and excellent chemoselectivity.
Figures



Similar articles
-
Mild bioconjugation through the oxidative coupling of ortho-aminophenols and anilines with ferricyanide.Angew Chem Int Ed Engl. 2014 Jan 20;53(4):1057-61. doi: 10.1002/anie.201307386. Epub 2013 Dec 5. Angew Chem Int Ed Engl. 2014. PMID: 24311449
-
Rapid chemoselective bioconjugation through oxidative coupling of anilines and aminophenols.J Am Chem Soc. 2011 Oct 19;133(41):16398-401. doi: 10.1021/ja2033298. Epub 2011 Sep 28. J Am Chem Soc. 2011. PMID: 21919497 Free PMC article.
-
Bioconjugation of gold nanoparticles through the oxidative coupling of ortho-aminophenols and anilines.Bioconjug Chem. 2014 Oct 15;25(10):1888-92. doi: 10.1021/bc5003746. Epub 2014 Oct 2. Bioconjug Chem. 2014. PMID: 25275488
-
Removal of amino groups from anilines through diazonium salt-based reactions.Org Biomol Chem. 2014 Sep 28;12(36):6965-71. doi: 10.1039/c4ob01286k. Org Biomol Chem. 2014. PMID: 25093920 Review.
-
Selective fluorogenic derivatization of 3-nitrotyrosine and 3,4-dihydroxyphenylalanine in peptides: a method designed for quantitative proteomic analysis.Methods Enzymol. 2008;441:19-32. doi: 10.1016/S0076-6879(08)01202-0. Methods Enzymol. 2008. PMID: 18554527 Review.
Cited by
-
Identification of signaling networks associated with lactate modulation of macrophages and dendritic cells.Heliyon. 2025 Jan 28;11(3):e42098. doi: 10.1016/j.heliyon.2025.e42098. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 39975831 Free PMC article.
-
Protein-Based Model for Energy Transfer between Photosynthetic Light-Harvesting Complexes Is Constructed Using a Direct Protein-Protein Conjugation Strategy.J Am Chem Soc. 2023 Jul 26;145(29):15827-15837. doi: 10.1021/jacs.3c02577. Epub 2023 Jul 12. J Am Chem Soc. 2023. PMID: 37438911 Free PMC article.
References
-
- Abello N, Kerstjens HA, Postma DS, Bischoff R. J Proteome Res. 2009;8:3222–3238. - PubMed
-
- Ischiropoulos H. Arch Biochem Biophys. 2009;484:117–121. - PubMed
-
- Souza JM, Peluffo G, Radi R. Free Radical Biol Med. 2008;45:357–366. - PubMed
-
- Beckman JS, Ischiropoulos H, Zhu L, van der Woerd M, Smith C, Chen J, Harrison J, Martin JC, Tsai M. Arch Biochem Biophys. 1992;298:438–445. - PubMed
-
- Vandervliet A, Eiserich JP, Oneill CA, Halliwell B, Cross CE. Arch Biochem Biophys. 1995;319:341–349. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

