Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module
- PMID: 27447450
- PMCID: PMC4961865
- DOI: 10.1038/ncomms12277
Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module
Abstract
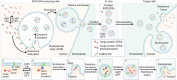
Nanoparticle-mediated delivery of functional macromolecules is a promising method for treating a variety of human diseases. Among nanoparticles, cell-derived exosomes have recently been highlighted as a new therapeutic strategy for the in vivo delivery of nucleotides and chemical drugs. Here we describe a new tool for intracellular delivery of target proteins, named 'exosomes for protein loading via optically reversible protein-protein interactions' (EXPLORs). By integrating a reversible protein-protein interaction module controlled by blue light with the endogenous process of exosome biogenesis, we are able to successfully load cargo proteins into newly generated exosomes. Treatment with protein-loaded EXPLORs is shown to significantly increase intracellular levels of cargo proteins and their function in recipient cells in vitro and in vivo. These results clearly indicate the potential of EXPLORs as a mechanism for the efficient intracellular transfer of protein-based therapeutics into recipient cells and tissues.
Conflict of interest statement
C.C. is the scientific founder and Chief Executive Officer of Cellex Life Sciences Inc. S.-W.R. and K.R.L. are employees of Cellex Life Sciences Inc. A patent application has been submitted by KAIST based on these results. The remaining authors declare no competing financial interests.
Figures
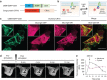
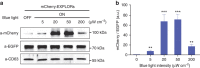

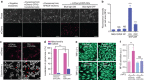
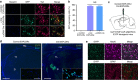
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

