Timely Endocytosis of Cytokinetic Enzymes Prevents Premature Spindle Breakage during Mitotic Exit
- PMID: 27447488
- PMCID: PMC4957831
- DOI: 10.1371/journal.pgen.1006195
Timely Endocytosis of Cytokinetic Enzymes Prevents Premature Spindle Breakage during Mitotic Exit
Abstract
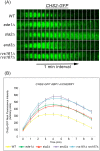
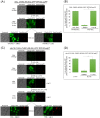
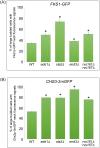
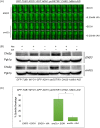
Cytokinesis requires the spatio-temporal coordination of membrane deposition and primary septum (PS) formation at the division site to drive acto-myosin ring (AMR) constriction. It has been demonstrated that AMR constriction invariably occurs only after the mitotic spindle disassembly. It has also been established that Chitin Synthase II (Chs2p) neck localization precedes mitotic spindle disassembly during mitotic exit. As AMR constriction depends upon PS formation, the question arises as to how chitin deposition is regulated so as to prevent premature AMR constriction and mitotic spindle breakage. In this study, we propose that cells regulate the coordination between spindle disassembly and AMR constriction via timely endocytosis of cytokinetic enzymes, Chs2p, Chs3p, and Fks1p. Inhibition of endocytosis leads to over accumulation of cytokinetic enzymes during mitotic exit, which accelerates the constriction of the AMR, and causes spindle breakage that eventually could contribute to monopolar spindle formation in the subsequent round of cell division. Intriguingly, the mitotic spindle breakage observed in endocytosis mutants can be rescued either by deleting or inhibiting the activities of, CHS2, CHS3 and FKS1, which are involved in septum formation. The findings from our study highlight the importance of timely endocytosis of cytokinetic enzymes at the division site in safeguarding mitotic spindle integrity during mitotic exit.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








References
-
- Irniger S, Piatti S, Michaelis C, Nasmyth K. Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell. 1995;81(2):269–78. Epub 1995/04/21. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

