Interaction between TNF and BmooMP-Alpha-I, a Zinc Metalloprotease Derived from Bothrops moojeni Snake Venom, Promotes Direct Proteolysis of This Cytokine: Molecular Modeling and Docking at a Glance
- PMID: 27447669
- PMCID: PMC4963855
- DOI: 10.3390/toxins8070223
Interaction between TNF and BmooMP-Alpha-I, a Zinc Metalloprotease Derived from Bothrops moojeni Snake Venom, Promotes Direct Proteolysis of This Cytokine: Molecular Modeling and Docking at a Glance
Abstract
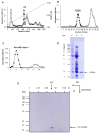
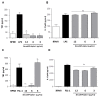
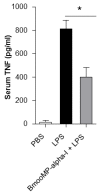
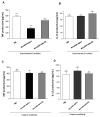
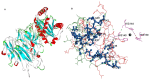
Tumor necrosis factor (TNF) is a major cytokine in inflammatory processes and its deregulation plays a pivotal role in several diseases. Here, we report that a zinc metalloprotease extracted from Bothrops moojeni venom (BmooMP-alpha-I) inhibits TNF directly by promoting its degradation. This inhibition was demonstrated by both in vitro and in vivo assays, using known TLR ligands. These findings are supported by molecular docking results, which reveal interaction between BmooMP-alpha-I and TNF. The major cluster of interaction between BmooMP-alpha-I and TNF was confirmed by the structural alignment presenting Ligand Root Mean Square Deviation LRMS = 1.05 Å and Interactive Root Mean Square Deviation IRMS = 1.01 Å, this result being compatible with an accurate complex. Additionally, we demonstrated that the effect of this metalloprotease on TNF is independent of cell cytotoxicity and it does not affect other TLR-triggered cytokines, such as IL-12. Together, these results indicate that this zinc metalloprotease is a potential tool to be further investigated for the treatment of inflammatory disorders involving TNF deregulation.
Keywords: BmooMP-alpha-I; TACE; TNF; zinc metalloprotease.
Figures



Similar articles
-
Treatment with a Zinc Metalloprotease Purified from Bothrops moojeni Snake Venom (BmooMP-Alpha-I) Reduces the Inflammation in an Experimental Model of Dextran Sulfate Sodium-Induced Colitis.Mediators Inflamm. 2019 Jul 29;2019:5195134. doi: 10.1155/2019/5195134. eCollection 2019. Mediators Inflamm. 2019. PMID: 31467484 Free PMC article.
-
Jararhagin, a hemorrhagic snake venom metalloproteinase from Bothrops jararaca.Toxicon. 2012 Sep 1;60(3):280-9. doi: 10.1016/j.toxicon.2012.03.026. Epub 2012 Apr 14. Toxicon. 2012. PMID: 22534074 Review.
-
Biochemical, pharmacological and structural characterization of BmooMP-I, a new P-I metalloproteinase from Bothrops moojeni venom.Biochimie. 2020 Dec;179:54-64. doi: 10.1016/j.biochi.2020.09.001. Epub 2020 Sep 16. Biochimie. 2020. PMID: 32946987
-
Moojenactivase, a novel pro-coagulant PIIId metalloprotease isolated from Bothrops moojeni snake venom, activates coagulation factors II and X and induces tissue factor up-regulation in leukocytes.Arch Toxicol. 2016 May;90(5):1261-78. doi: 10.1007/s00204-015-1533-6. Epub 2015 May 31. Arch Toxicol. 2016. PMID: 26026608
-
Jararhagin and its multiple effects on hemostasis.Toxicon. 2005 Jun 15;45(8):987-96. doi: 10.1016/j.toxicon.2005.02.013. Epub 2005 Apr 2. Toxicon. 2005. PMID: 15922770 Review.
Cited by
-
Bothrops moojeni Venom and Its Components Strongly Affect Osteoclasts' Maturation and Protein Patterns.Toxins (Basel). 2021 Jun 30;13(7):459. doi: 10.3390/toxins13070459. Toxins (Basel). 2021. PMID: 34208941 Free PMC article.
-
Stress-Induced Mucus Secretion and Its Composition by a Combination of Proteomics and Metabolomics of the Jellyfish Aurelia coerulea.Mar Drugs. 2018 Sep 18;16(9):341. doi: 10.3390/md16090341. Mar Drugs. 2018. PMID: 30231483 Free PMC article.
-
Antivenom Production against Bothrops jararaca and Bothrops erythromelas Snake Venoms Using Cross-Linked Chitosan Nanoparticles as an Immunoadjuvant.Toxins (Basel). 2018 Apr 16;10(4):158. doi: 10.3390/toxins10040158. Toxins (Basel). 2018. PMID: 29659491 Free PMC article.
-
Ileal inflammation is reduced due to treatment with a metalloprotease from BmooMP-α-I snake venom in an experimental model of Toxoplasma gondii infection.Parasitol Res. 2023 Dec 22;123(1):65. doi: 10.1007/s00436-023-08033-9. Parasitol Res. 2023. PMID: 38133827
-
Harnessing the Power of Venomous Animal-Derived Toxins against COVID-19.Toxins (Basel). 2023 Feb 14;15(2):159. doi: 10.3390/toxins15020159. Toxins (Basel). 2023. PMID: 36828473 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

