Impact of Masked Replacement of Sugar-Sweetened with Sugar-Free Beverages on Body Weight Increases with Initial BMI: Secondary Analysis of Data from an 18 Month Double-Blind Trial in Children
- PMID: 27447721
- PMCID: PMC4957753
- DOI: 10.1371/journal.pone.0159771
Impact of Masked Replacement of Sugar-Sweetened with Sugar-Free Beverages on Body Weight Increases with Initial BMI: Secondary Analysis of Data from an 18 Month Double-Blind Trial in Children
Abstract
Background: Substituting sugar-free for sugar-sweetened beverages reduces weight gain. This effect may be more pronounced in children with a high body mass index (BMI) because their sensing of kilocalories might be compromised. We investigated the impact of sugar-free versus sugary drinks separately in children with a higher and a lower initial BMI z score, and predicted caloric intakes and degree of compensation in the two groups.
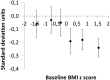
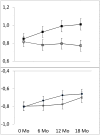
Methods and findings: This is a secondary, explorative analysis of our double-blind randomized controlled trial (RCT) which showed that replacement of one 250-mL sugary drink per day by a sugar-free drink for 18 months significantly reduced weight gain. In the 477 children who completed the trial, mean initial weights were close to the Dutch average. Only 16% were overweight and 3% obese. Weight changes were expressed as BMI z-score, i.e. as standard deviations of the BMI distribution per age and sex group. We designated the 239 children with an initial BMI z-score below the median as 'lower BMI' and the 238 children above the median as 'higher BMI'. The difference in caloric intake from experimental beverages between treatments was 86 kcal/day both in the lower and in the higher BMI group. We used a multiple linear regression and the coefficient of the interaction term (initial BMI group times treatment), indicated whether children with a lower BMI responded differently from children with a higher BMI. Statistical significance was defined as p ≤ 0.05. Relative to the sugar sweetened beverage, consumption of the sugar-free beverage for 18 months reduced the BMI z-score by 0.05 SD units within the lower BMI group and by 0.21 SD within the higher BMI group. Body weight gain was reduced by 0.62 kg in the lower BMI group and by 1.53 kg in the higher BMI group. Thus the treatment reduced the BMI z-score by 0.16 SD units more in the higher BMI group than in the lower BMI group (p = 0.04; 95% CI -0.31 to -0.01). The impact of the intervention on body weight gain differed by 0.90 kg between BMI groups (p = 0.09; 95% CI -1.95 to 0.14). In addition, we used a physiologically-based model of growth and energy balance to estimate the degree to which children had compensated for the covertly removed sugar kilocalories by increasing their intake of other foods. The model predicts that children with a lower BMI had compensated 65% (95% CI 28 to 102) of the covertly removed sugar kilocalories, whereas children with a higher BMI compensated only 13% (95% CI -37 to 63).
Conclusions: The children with a BMI above the median might have a reduced tendency to compensate for changes in caloric intake. Differences in these subconscious compensatory mechanisms may be an important cause of differences in the tendency to gain weight. If further research bears this out, cutting down on the intake of sugar-sweetened drinks may benefit a large proportion of children, especially those who show a tendency to become overweight.
Trial registration: ClinicalTrials.gov NCT00893529.
Conflict of interest statement
Figures
References
-
- DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite. 2005;44: 187–193. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

