Comparative analysis of metallic nanoparticles as exogenous soft tissue contrast for live in vivo micro-computed tomography imaging of avian embryonic morphogenesis
- PMID: 27447729
- PMCID: PMC5026601
- DOI: 10.1002/dvdy.24433
Comparative analysis of metallic nanoparticles as exogenous soft tissue contrast for live in vivo micro-computed tomography imaging of avian embryonic morphogenesis
Abstract
Background: Gestationally survivable congenital malformations arise during mid-late stages of development that are inaccessible in vivo with traditional optical imaging for assessing long-term abnormal patterning. MicroCT is an attractive technology to rapidly and inexpensively generate quantitative three-dimensional (3D) datasets but requires exogenous contrast media. Here we establish dose-dependent toxicity, persistence, and biodistribution of three different metallic nanoparticles in day 4 chick embryos.
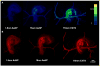
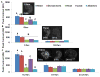
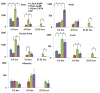
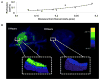
Results: We determined that 110-nm alkaline earth metal particles were nontoxic and persisted in the chick embryo for up to 24 hr postinjection with contrast enhancement levels at high as 1,600 Hounsfield units (HU). The 15-nm gold nanoparticles persisted with x-ray attenuation higher than that of the surrounding yolk and albumen for up to 8 hr postinjection, while 1.9-nm particles resulted in lethality by 8 hr. We identified spatial and temporally heterogeneous contrast enhancement ranging from 250 to 1,600 HU. With the most optimal 110-nm alkaline earth metal particles, we quantified an exponential increase in the tissue perfusion vs. distance from the dorsal aorta into the flank over 8 hr with a peak perfusion rate of 0.7 μm(2) /s measured at a distance of 0.3 mm.
Conclusions: These results demonstrate the safety, efficacy, and opportunity of nanoparticle based contrast media in live embryos for quantitative analysis of embryogenesis. Developmental Dynamics 245:1001-1010, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: biodistribution; development; dorsal aorta; embryo; nanoparticles; perfusion; toxicity.
© 2016 Wiley Periodicals, Inc.
Figures


References
-
- Aillon KL, Xie Y, El-Gendy N, Berkland CJ, Forrest ML. Effects of nanomaterial physicochemical properties on in vivo toxicity. Advanced Drug Delivery Reviews. 2009;61(6):457–466. http://doi.org/10.1016/j.addr.2009.03.010. - DOI - PMC - PubMed
-
- Ashton JR, Clark DP, Moding EJ, Ghaghada K, Kirsch DG, West JL, Badea CT. Dual-energy micro-CT functional imaging of primary lung cancer in mice using gold and iodine nanoparticle contrast agents: a validation study. PloS One. 2014;9(2):e88129. http://doi.org/10.1371/journal.pone.0088129. - DOI - PMC - PubMed
-
- Badea C, D M, Holdsworth D, Johnson G, Angiography S. In vivo small animal imaging using micro-CT and digital subtraction angiography. Physics in Medicine and Biology. 2008;53(19):1–36. http://doi.org/10.1088/0031-9155/53/19/R01.In. - DOI - PMC - PubMed
-
- Bellairs R, Osmond M. Atlas of Chick Development. 2014 http://doi.org/10.1016/B978-0-12-384951-9.00013-7. - DOI
-
- Boll H, Nittka S, Doyon F, Neumaier M, Marx A, Kramer M, Brockmann Ma, et al. Micro-CT based experimental liver imaging using a nanoparticulate contrast agent: a longitudinal study in mice. PloS One. 2011;6(9):e25692. http://doi.org/10.1371/journal.pone.0025692. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

