Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial
- PMID: 27447966
- PMCID: PMC5312408
- DOI: 10.18632/oncotarget.10607
Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial
Abstract
Background: No standard chemotherapy is used as neoadjuvant therapy in triple negative breast cancer (TNBC). This study has compared carboplatin plus paclitaxel with commonly used epirubicin plus paclitaxel as neoadjuvant chemotherapy (NAC) in TNBC.
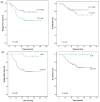
Results: 91 patients with a median age of 47 years (PC 47 patients, EP 44 patients) were enrolled. 65% of the patients were premenopausal. While the objective response rate was similar in the PC and EP arm (89.4% vs. 79.5%, P = 0.195), the pCR rate in the PC arm was significantly higher (38.6% vs. 14.0%, P = 0.014). The median follow-up time was 55.0 months. 5-year RFS were 77.6% and 56.2%, significantly higher in the PC arm, P = 0.043. No significant difference in OS was observed between the two arms (P = 0.350). Adverse events were similar, except for more thrombocytopenia in the PC arm (P = 0.001).
Methods: Patients with stage II/III TNBC were randomized to receive either paclitaxel (175 mg/m2, day1) plus carboplatin (Area Under the Curve = 5, day2) (PC) or epirubicin (75mg/m2, day1) plus paclitaxel (175 mg/m2, day2) (EP) as NAC every three weeks for 4-6 cycles. The primary endpoint was rate of pathologic complete response (pCR).The secondary endpoints included relapse-free survival (RFS), overall survival (OS) and safety.
Conclusions: This study suggested that the addition of carboplatin to paclitaxel was superior to the regimen of epirubicin plus paclitaxel as NAC for TNBC in terms of improving pCR rate and RFS. Further phase 3 study has already started.
Keywords: breast cancer; carboplatin; neoadjuvant chemotherapy; paclitaxel; triple negative.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures


References
-
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. doi: 10.1002/cncr.22618. - DOI - PubMed
-
- Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. doi: 10.1200/jco.2007.14.4147. - DOI - PubMed
-
- Huober J, von Minckwitz G, Denkert C, Tesch H, Weiss E, Zahm DM, Belau A, Khandan F, Hauschild M, Thomssen C, Hogel B, Darb-Esfahani S, Mehta K, et al. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat. 2010;124:133–40. doi: 10.1007/s10549-010-1103-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

