Micro-dose hCG as luteal phase support without exogenous progesterone administration: mathematical modelling of the hCG concentration in circulation and initial clinical experience
- PMID: 27448021
- PMCID: PMC5065547
- DOI: 10.1007/s10815-016-0764-7
Micro-dose hCG as luteal phase support without exogenous progesterone administration: mathematical modelling of the hCG concentration in circulation and initial clinical experience
Abstract
For the last two decades, exogenous progesterone administration has been used as luteal phase support (LPS) in connection with controlled ovarian stimulation combined with use of the human chorionic gonadotropin (hCG) trigger for the final maturation of follicles. The introduction of the GnRHa trigger to induce ovulation showed that exogenous progesterone administration without hCG supplementation was insufficient to obtain satisfactory pregnancy rates. This has prompted development of alternative strategies for LPS. Augmenting the local endogenous production of progesterone by the multiple corpora lutea has been one focus with emphasis on one hand to avoid development of ovarian hyper-stimulation syndrome and, on the other hand, to provide adequate levels of progesterone to sustain implantation. The present study evaluates the use of micro-dose hCG for LPS support and examines the potential advances and disadvantages. Based on the pharmacokinetic characteristics of hCG, the mathematical modelling of the concentration profiles of hCG during the luteal phase has been evaluated in connection with several different approaches for hCG administration as LPS. It is suggested that the currently employed LPS provided in connection with the GnRHa trigger (i.e. 1.500 IU) is too strong, and that daily micro-dose hCG administration is likely to provide an optimised LPS with the current available drugs. Initial clinical results with the micro-dose hCG approach are presented.
Keywords: Corpus luteum function; GnRH agonist trigger; Luteal phase support; hCG.
Conflict of interest statement
The authors declare that they have no conflict of interest Ethical approval For this type of study, formal consent is not required.
Figures

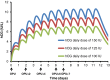
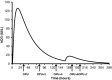

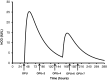

References
-
- Fauser BC, De Jong D, Loivennes F, Wramsby H, Tay C, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist Ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:709–15. doi: 10.1210/jcem.87.2.8197. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

