BipA Is Associated with Preventing Autoagglutination and Promoting Biofilm Formation in Bordetella holmesii
- PMID: 27448237
- PMCID: PMC4957798
- DOI: 10.1371/journal.pone.0159999
BipA Is Associated with Preventing Autoagglutination and Promoting Biofilm Formation in Bordetella holmesii
Abstract
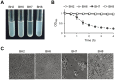
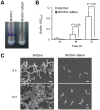
Bordetella holmesii causes both invasive and respiratory diseases in humans. Although the number of cases of pertussis-like respiratory illnesses due to B. holmesii infection has increased in the last decade worldwide, little is known about the virulence factors of the organism. Here, we analyzed a B. holmesii isolate that forms large aggregates and precipitates in suspension, and subsequently demonstrated that the autoagglutinating isolate is deficient in Bordetella intermediate protein A (BipA) and that this deletion is caused by a frame-shift mutation in the bipA gene. A BipA-deficient mutant generated by homologous recombination also exhibited the autoagglutination phenotype. Moreover, the BipA mutant adhered poorly to an abiotic surface and failed to form biofilms, as did two other B. holmesii autoagglutinating strains, ATCC 51541 and ATCC 700053, which exhibit transcriptional down-regulation of bipA gene expression, indicating that autoagglutination indirectly inhibits biofilm formation. In a mouse intranasal infection model, the BipA mutant showed significantly lower levels of initial lung colonization than did the parental strain (P < 0.01), suggesting that BipA might be a critical virulence factor in B. holmesii respiratory infection. Together, our findings suggest that BipA production plays an essential role in preventing autoagglutination and indirectly promoting biofilm formation by B. holmesii.
Conflict of interest statement
Figures





Similar articles
-
Development of vaccines against pertussis caused by Bordetella holmesii using a mouse intranasal challenge model.Microbiol Immunol. 2016 Sep;60(9):599-608. doi: 10.1111/1348-0421.12409. Microbiol Immunol. 2016. PMID: 27515393
-
Differential Bvg phase-dependent regulation and combinatorial role in pathogenesis of two Bordetella paralogs, BipA and BcfA.J Bacteriol. 2007 May;189(10):3695-704. doi: 10.1128/JB.00009-07. Epub 2007 Mar 9. J Bacteriol. 2007. PMID: 17351043 Free PMC article.
-
Identification and characterization of BipA, a Bordetella Bvg-intermediate phase protein.Mol Microbiol. 2001 Jan;39(1):65-78. doi: 10.1046/j.1365-2958.2001.02191.x. Mol Microbiol. 2001. PMID: 11123689
-
Bordetella holmesii infection: current knowledge and a vision for future research.Expert Rev Anti Infect Ther. 2015 Aug;13(8):965-71. doi: 10.1586/14787210.2015.1056161. Epub 2015 Jun 11. Expert Rev Anti Infect Ther. 2015. PMID: 26065696 Review.
-
Bordetella holmesii: an under-recognised Bordetella species.Lancet Infect Dis. 2014 Jun;14(6):510-9. doi: 10.1016/S1473-3099(14)70021-0. Epub 2014 Apr 7. Lancet Infect Dis. 2014. PMID: 24721229 Review.
Cited by
-
In vivo evolution of an emerging zoonotic bacterial pathogen in an immunocompromised human host.Nat Commun. 2021 Jul 23;12(1):4495. doi: 10.1038/s41467-021-24668-7. Nat Commun. 2021. PMID: 34301946 Free PMC article.
-
BipA exerts temperature-dependent translational control of biofilm-associated colony morphology in Vibrio cholerae.Elife. 2021 Feb 12;10:e60607. doi: 10.7554/eLife.60607. Elife. 2021. PMID: 33588990 Free PMC article.
-
Bacterial autoaggregation.AIMS Microbiol. 2018 Mar 1;4(1):140-164. doi: 10.3934/microbiol.2018.1.140. eCollection 2018. AIMS Microbiol. 2018. PMID: 31294207 Free PMC article. Review.
-
Bordetella holmesii: Lipid A Structures and Corresponding Genomic Sequences Comparison in Three Clinical Isolates and the Reference Strain ATCC 51541.Int J Mol Sci. 2017 May 18;18(5):1080. doi: 10.3390/ijms18051080. Int J Mol Sci. 2017. PMID: 28524084 Free PMC article.
-
In vivo RNA-seq and infection model reveal the different infection and immune characteristics of B. pertussis strains in China.Front Cell Infect Microbiol. 2025 Jun 11;15:1547751. doi: 10.3389/fcimb.2025.1547751. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40568708 Free PMC article.
References
-
- Shepard CW, Daneshvar MI, Kaiser RM, Ashford DA, Lonsway D, Patel JB, et al. Bordetella holmesii bacteremia: a newly recognized clinical entity among asplenic patients. Clin Infect Dis. 2004;38:799–804. - PubMed
-
- Tang YW, Hopkins MK, Kolbert CP, Hartley PA, Severance PJ, Persing DH. Bordetella holmesii-like organisms associated with septicemia, endocarditis, and respiratory failure. Clin Infect Dis. 1998;26:389–392. - PubMed
-
- Njamkepo E, Delisle F, Hagege I, Gerbaud G, Guiso N. Bordetella holmesii isolated from a patient with sickle cell anemia: analysis and comparison with other Bordetella holmesii isolates. Clin Microbiol Infect. 2000;6:131–136. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

