Therapeutic potential of adipose stem cell-derived conditioned medium against pulmonary hypertension and lung fibrosis
- PMID: 27448286
- PMCID: PMC5275771
- DOI: 10.1111/bph.13562
Therapeutic potential of adipose stem cell-derived conditioned medium against pulmonary hypertension and lung fibrosis
Abstract
Background and purpose: Pulmonary hypertension (PH) and pulmonary fibrosis (PF) are life threatening cardiopulmonary diseases. Existing pharmacological interventions have failed to improve clinical outcomes or reduce disease-associated mortality. Emerging evidence suggests that stem cells offer an effective treatment approach against various pathological conditions. It has been proposed that their beneficial actions may be mediated via secretion of paracrine factors. Herein, we evaluated the therapeutic potential of conditioned media (CM) from adipose stem cells (ASCs) against experimental models of PH and PF.
Experimental approach: Monocrotaline (MCT) or bleomycin (Bleo) was injected into male Sprague-Dawley rats to induce PH or PF respectively. A subset of MCT and Bleo animals were treated with ASCs or CM. Echocardiographic and haemodynamic measurements were performed at the end of the study. Lung and heart tissues were harvested for RNA, protein and histological measurements.
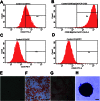
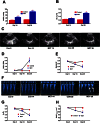
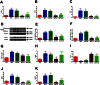
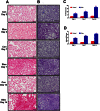
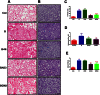
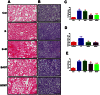
Key results: CM treatment attenuated MCT-induced PH by improving pulmonary blood flow and inhibiting cardiac remodelling. Further, histological studies revealed that right ventricular fibrosis, pulmonary vessel wall thickness and pericyte distribution were significantly decreased by CM administration. Likewise, CM therapy arrested the progression of PF in the Bleo model by reducing collagen deposition. Elevated expression of markers associated with tissue remodelling and inflammation were significantly reduced in both PF and PH lungs. Similar results were obtained with ASCs administration.
Conclusions and implications: Our study indicates that CM treatment is as effective as ASCs in treating PH and PF. These beneficial effects of CM may provide an innovative approach to treat cardiopulmonary disorders.
© 2016 The British Pharmacological Society.
Figures



References
-
- Akhavein F, St‐Michel EJ, Seifert E, Rohlicek CV (2007). Decreased left ventricular function, myocarditis, and coronary arteriolar medial thickening following monocrotaline administration in adult rats. J Appl Physiol (1985) 103: 287–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

