Proteins that mediate protein aggregation and cytotoxicity distinguish Alzheimer's hippocampus from normal controls
- PMID: 27448508
- PMCID: PMC5013017
- DOI: 10.1111/acel.12501
Proteins that mediate protein aggregation and cytotoxicity distinguish Alzheimer's hippocampus from normal controls
Abstract
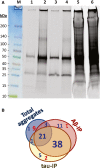
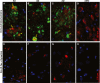
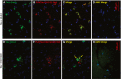
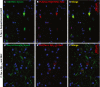
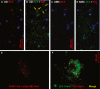
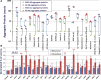
Neurodegenerative diseases are distinguished by characteristic protein aggregates initiated by disease-specific 'seed' proteins; however, roles of other co-aggregated proteins remain largely unexplored. Compact hippocampal aggregates were purified from Alzheimer's and control-subject pools using magnetic-bead immunoaffinity pulldowns. Their components were fractionated by electrophoretic mobility and analyzed by high-resolution proteomics. Although total detergent-insoluble aggregates from Alzheimer's and controls had similar protein content, within the fractions isolated by tau or Aβ1-42 pulldown, the protein constituents of Alzheimer-derived aggregates were more abundant, diverse, and post-translationally modified than those from controls. Tau- and Aβ-containing aggregates were distinguished by multiple components, and yet shared >90% of their protein constituents, implying similar accretion mechanisms. Alzheimer-specific protein enrichment in tau-containing aggregates was corroborated for individuals by three analyses. Five proteins inferred to co-aggregate with tau were confirmed by precise in situ methods, including proximity ligation amplification that requires co-localization within 40 nm. Nematode orthologs of 21 proteins, which showed Alzheimer-specific enrichment in tau-containing aggregates, were assessed for aggregation-promoting roles in C. elegans by RNA-interference 'knockdown'. Fifteen knockdowns (71%) rescued paralysis of worms expressing muscle Aβ, and 12 (57%) rescued chemotaxis disrupted by neuronal Aβ expression. Proteins identified in compact human aggregates, bound by antibody to total tau, were thus shown to play causal roles in aggregation based on nematode models triggered by Aβ1-42 . These observations imply shared mechanisms driving both types of aggregation, and/or aggregate-mediated cross-talk between tau and Aβ. Knowledge of protein components that promote protein accrual in diverse aggregate types implicates common mechanisms and identifies novel targets for drug intervention.
Keywords: Abeta(1-42); Alzheimer (Disease); C. elegans; acetylation (protein); aggregation (protein); beta amyloid; microtubule-associated protein tau; neurodegeneration; neurotoxicity; oxidation (protein); phosphorylation (protein); proteomics.
© 2016 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures
References
-
- Abdel‐Salam OM (2014) The paths to neurodegeneration in genetic Parkinson's disease. CNS Neurol. Disord. Drug Targets 13, 1485–1512. - PubMed
-
- Ahn EH, Kim DW, Shin MJ, Kim HR, Kim SM, Woo SJ, Eom SA, Jo HS, Kim DS, Cho SW, Park J, Eum WS, Choi SY (2014) PEP‐1‐PEA‐15 protects against toxin‐induced neuronal damage in a mouse model of Parkinson's disease. Biochim. Biophys. Acta 1840, 1686–1700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

