Adverse events following pandemic influenza A (H1N1) 2009 monovalent and seasonal influenza vaccinations during the 2009-2010 season in the active component U.S. military and civilians aged 17-44years reported to the Vaccine Adverse Event Reporting System
- PMID: 27449076
- PMCID: PMC6463880
- DOI: 10.1016/j.vaccine.2016.07.019
Adverse events following pandemic influenza A (H1N1) 2009 monovalent and seasonal influenza vaccinations during the 2009-2010 season in the active component U.S. military and civilians aged 17-44years reported to the Vaccine Adverse Event Reporting System
Abstract
Background: No comparative review of Vaccine Adverse Event Reporting System (VAERS) submissions following pandemic influenza A (H1N1) 2009 and seasonal influenza vaccinations during the pandemic season among U.S. military personnel has been published.
Methods: We compared military vs. civilian adverse event reporting rates. Adverse events (AEs) following vaccination were identified from VAERS for adults aged 17-44years after pandemic (monovalent influenza [MIV], and seasonal (trivalent inactivated influenza [IIV3], live attenuated influenza [LAIV3]) vaccines. Military vaccination coverage was provided by the Department of Defense's Defense Medical Surveillance System. Civilian vaccination coverage was estimated using data from the National 2009 H1N1 Flu Survey and the Behavioral Risk Factor Surveillance System survey.
Results: Vaccination coverage was more than four times higher for MIV and more than twenty times higher for LAIV3 in the military than in the civilian population. The reporting rate of serious AE reports following MIV in service personnel (1.19 per 100,000) was about half that reported by the civilian population (2.45 per 100,000). Conversely, the rate of serious AE reports following LAIV3 among service personnel (1.32 per 100,000) was more than twice that of the civilian population. Although fewer military AEs following MIV were reported overall, the rate of Guillain-Barré Syndrome (GBS) (4.01 per million) was four times greater than that in the civilian population. (1.04 per million).
Conclusions: Despite higher vaccination coverage in service personnel, the rate of serious AEs following MIV was about half that in civilians. The rate of GBS reported following MIV was higher in the military.
Keywords: Pandemic influenza A H1N1 (2009) influenza vaccine; Vaccine safety.
Published by Elsevier Ltd.
Conflict of interest statement
Conflict of interest
None of the authors has conflicts of interest.
Figures
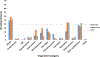
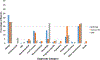
Similar articles
-
A cluster of nonspecific adverse events in a military reserve unit following pandemic influenza A (H1N1) 2009 vaccination-possible stimulated reporting?Vaccine. 2012 Mar 23;30(14):2421-6. doi: 10.1016/j.vaccine.2012.01.072. Epub 2012 Feb 3. Vaccine. 2012. PMID: 22310205
-
Safety of influenza A (H1N1) 2009 monovalent vaccines - United States, October 1-November 24, 2009.MMWR Morb Mortal Wkly Rep. 2009 Dec 11;58(48):1351-6. MMWR Morb Mortal Wkly Rep. 2009. PMID: 20010511
-
Post-licensure surveillance of trivalent live attenuated influenza vaccine in adults, United States, Vaccine Adverse Event Reporting System (VAERS), July 2005-June 2013.Vaccine. 2014 Nov 12;32(48):6499-504. doi: 10.1016/j.vaccine.2014.09.018. Epub 2014 Sep 22. Vaccine. 2014. PMID: 25258101
-
Post-licensure surveillance of quadrivalent inactivated influenza (IIV4) vaccine in the United States, Vaccine Adverse Event Reporting System (VAERS), July 1, 2013-May 31, 2015.Vaccine. 2016 May 11;34(22):2507-12. doi: 10.1016/j.vaccine.2016.03.048. Epub 2016 Mar 23. Vaccine. 2016. PMID: 27015735 Free PMC article. Review.
-
Monitoring the safety of high-dose, trivalent inactivated influenza vaccine in the vaccine adverse event reporting system (VAERS), 2011 - 2019.Vaccine. 2020 Aug 18;38(37):5923-5926. doi: 10.1016/j.vaccine.2020.07.007. Epub 2020 Jul 21. Vaccine. 2020. PMID: 32709434 Review.
Cited by
-
The reporting sensitivity of the Vaccine Adverse Event Reporting System (VAERS) for anaphylaxis and for Guillain-Barré syndrome.Vaccine. 2020 Nov 3;38(47):7458-7463. doi: 10.1016/j.vaccine.2020.09.072. Epub 2020 Oct 7. Vaccine. 2020. PMID: 33039207 Free PMC article.
-
Enhanced passive safety surveillance of a quadrivalent inactivated split virion influenza vaccine in Finland during the influenza season 2020/21.BMC Public Health. 2022 Aug 8;22(1):1506. doi: 10.1186/s12889-022-13898-z. BMC Public Health. 2022. PMID: 35941631 Free PMC article.
-
Role of anti-polyethylene glycol (PEG) antibodies in the allergic reactions to PEG-containing Covid-19 vaccines: Evidence for immunogenicity of PEG.Vaccine. 2023 Jul 12;41(31):4561-4570. doi: 10.1016/j.vaccine.2023.06.009. Epub 2023 Jun 5. Vaccine. 2023. PMID: 37330369 Free PMC article.
-
Antigenic Variability a Potential Factor in Assessing Relationship Between Guillain Barré Syndrome and Influenza Vaccine - Up to Date Literature Review.Cureus. 2020 Sep 2;12(9):e10208. doi: 10.7759/cureus.10208. Cureus. 2020. PMID: 33033684 Free PMC article. Review.
-
Enhanced Safety Surveillance of Seasonal Quadrivalent Influenza Vaccines in English Primary Care: Interim Analysis.Adv Ther. 2018 Aug;35(8):1199-1214. doi: 10.1007/s12325-018-0747-4. Epub 2018 Jul 11. Adv Ther. 2018. PMID: 29995300 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Safety of influenza A (H1N1) 2009 monovalent vaccines – United States, October 1-November 24, 2009. MMWR Morbidity and mortality weekly report, vol. 58, 2009, pp. 1351–1356. - PubMed
-
- Mayet A, Ligier C, Gache K, Manet G, Nivoix P, Dia A, et al. Adverse events following pandemic influenza vaccine Pandemrix(R) reported in the French military forces–2009–2010. Vaccine 2011;29:2576–81. - PubMed
-
- Banzhoff A, Haertel S, Praus M. Passive surveillance of adverse events of an MF59-adjuvanted H1N1v vaccine during the pandemic mass vaccinations. Human Vaccines 2011;7:539–48. - PubMed
-
- Vellozzi C, Broder KR, Haber P, Guh A, Nguyen M, Cano M, et al. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009-January 31, 2010. Vaccine 2010;28:7248–55. - PubMed
-
- Halsey NA, Griffioen M, Dreskin SC, Dekker CL, Wood R, Sharma D, et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: reports to VAERS. Vaccine 2013;31:6107–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

