IL-1β-stimulated β-catenin up-regulation promotes angiogenesis in human lung-derived mesenchymal stromal cells through a NF-κB-dependent microRNA-433 induction
- PMID: 27449086
- PMCID: PMC5312322
- DOI: 10.18632/oncotarget.10683
IL-1β-stimulated β-catenin up-regulation promotes angiogenesis in human lung-derived mesenchymal stromal cells through a NF-κB-dependent microRNA-433 induction
Abstract
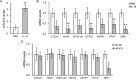
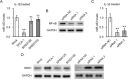
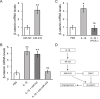
Considerable attentions have been focused on the treatment of lung injury using mesenchymal stem cells that can replenish damaged tissues including the blood vessels. In human lung-derived mesenchymal stem cells (hL-MSC), we investigated the potential role of an IL-1β-stimulated miR-433 pathway in angiogenesis in vitro. The expressions of miR-433 and its target genes were examined in cells treated with IL-1β. The angiogenic activity of hL-MSC was studied by cell migration and tube formation assays in which miR-433 levels were manipulated. The reporter assay and chromatin immunoprecipitation (ChIP) were also performed to analyze the underlying regulations. We found that the expression of miR-433 was enhanced in hL-MSC by IL-1β in a NF-κB dependent manner via a NF-κB binding site at its promoter region. The effects of IL-1β on promoting angiogenic activities in hL-MSC can be mimicked by the overexpression of miR-433 and were blocked by anti-miR-433. Mechanistically, our data suggested that miR-433 directly targets the 3'-UTR of Dickkopf Wnt signaling pathway inhibitor 1 (DKK1) mRNA and decreases its expression. Consistently, the expression of β-catenin, the major mediator of canonical Wnt pathway that is capable of inducing endothelial differentiation and angiogenesis, was upregulated by IL-1β through miR-433. Thus, increasing miR-433 expression by IL-1β in mesenchymal stem cells could stimulate their capacity of vascular remodeling for efficient repair processes, which may be utilized as a therapeutic target in patients suffering from severe lung injury.
Keywords: Wnt/β-catenin; angiogenesis; lung injury; mesenchymal stem cell; microRNA.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Avecillas JF, Freire AX, Arroliga AC. Clinical epidemiology of acute lung injury and acute respiratory distress syndrome: incidence diagnosis and outcomes. Clin Chest Med. 2006;27:549–557. abstract vii. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. - PubMed
-
- D'Agostino B, Sullo N, Siniscalco D, De Angelis A, Rossi F. Mesenchymal stem cell therapy for the treatment of chronic obstructive pulmonary disease. Expert Opin Biol Ther. 2010;10:681–687. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

