Dichloroacetate and metformin synergistically suppress the growth of ovarian cancer cells
- PMID: 27449090
- PMCID: PMC5312324
- DOI: 10.18632/oncotarget.10694
Dichloroacetate and metformin synergistically suppress the growth of ovarian cancer cells
Abstract
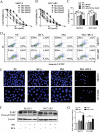
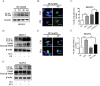
Both dichloroacetate (DCA) and metformin (Met) have shown promising antitumor efficacy by regulating cancer cell metabolism. However, the DCA-mediated protective autophagy and Met-induced lactate accumulation limit their tumor-killing potential respectively. So overcoming the corresponding shortages will improve their therapeutic effects. In the present study, we found that DCA and Met synergistically inhibited the growth and enhanced the apoptosis of ovarian cancer cells. Interestingly, we for the first time revealed that Met sensitized DCA via dramatically attenuating DCA-induced Mcl-1 protein and protective autophagy, while DCA sensitized Met through markedly alleviating Met-induced excessive lactate accumulation and glucose consumption. The in vivo experiments in nude mice also showed that DCA and Met synergistically suppressed the growth of xenograft ovarian tumors. These results may pave a way for developing novel strategies for the treatment of ovarian cancer based on the combined use of DCA and Met.
Keywords: Mcl-1; cancer metabolism; dichloroacetate; metformin; ovarian cancer.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures




Similar articles
-
Dichloroacetate enhances the anti-tumor effect of sorafenib via modulating the ROS-JNK-Mcl-1 pathway in liver cancer cells.Exp Cell Res. 2021 Sep 1;406(1):112755. doi: 10.1016/j.yexcr.2021.112755. Epub 2021 Jul 29. Exp Cell Res. 2021. PMID: 34332981
-
Metformin enhances antitumor action of sodium dichloroacetate against glioma C6.Exp Oncol. 2019 Jun;41(2):123-129. doi: 10.32471/exp-oncology.2312-8852.vol-41-no-2.13064. Exp Oncol. 2019. PMID: 31262158
-
Metformin enhances cytotoxic action of dichloroacetate against Lewis lung carcinoma cells in vitro.Exp Oncol. 2020 Mar;42(1):35-39. doi: 10.32471/exp-oncology.2312-8852.vol-42-no-1.14318. Exp Oncol. 2020. PMID: 32231186
-
Dichloroacetate (DCA) and Cancer: An Overview towards Clinical Applications.Oxid Med Cell Longev. 2019 Nov 14;2019:8201079. doi: 10.1155/2019/8201079. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31827705 Free PMC article. Review.
-
Dichloroacetate and cancer: new home for an orphan drug?Biochim Biophys Acta. 2014 Dec;1846(2):617-29. doi: 10.1016/j.bbcan.2014.08.005. Epub 2014 Aug 23. Biochim Biophys Acta. 2014. PMID: 25157892 Review.
Cited by
-
Inflammatory microenvironment of fibrotic liver promotes hepatocellular carcinoma growth, metastasis and sorafenib resistance through STAT3 activation.J Cell Mol Med. 2021 Feb;25(3):1568-1582. doi: 10.1111/jcmm.16256. Epub 2021 Jan 7. J Cell Mol Med. 2021. PMID: 33410581 Free PMC article.
-
Inhibition of HIF1α and PDK Induces Cell Death of Glioblastoma Multiforme.Exp Neurobiol. 2017 Oct;26(5):295-306. doi: 10.5607/en.2017.26.5.295. Epub 2017 Oct 25. Exp Neurobiol. 2017. PMID: 29093638 Free PMC article.
-
Metformin as Potential Therapy for High-Grade Glioma.Cancers (Basel). 2020 Jan 15;12(1):210. doi: 10.3390/cancers12010210. Cancers (Basel). 2020. PMID: 31952173 Free PMC article.
-
Metabolic Plasticity of Tumor Cells: How They Do Adapt to Food Deprivation.Adv Exp Med Biol. 2020;1219:109-123. doi: 10.1007/978-3-030-34025-4_6. Adv Exp Med Biol. 2020. PMID: 32130696 Review.
-
Metformin sensitizes endometrial cancer cells to chemotherapy through IDH1-induced Nrf2 expression via an epigenetic mechanism.Oncogene. 2018 Oct;37(42):5666-5681. doi: 10.1038/s41388-018-0360-7. Epub 2018 Jun 19. Oncogene. 2018. PMID: 29921847
References
-
- Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. - PubMed
-
- Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey PJ, Kassahn KS, Newell F, Quinn MC, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. - PubMed
-
- Fan JY, Yang Y, Xie JY, Lu YL, Shi K, Huang YQ. MicroRNA-144 mediates metabolic shift in ovarian cancer cells by directly targeting Glut1. Tumour Biol. 2015 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

