Short-term pyrrolidine dithiocarbamate administration attenuates cachexia-induced alterations to muscle and liver in ApcMin/+ mice
- PMID: 27449092
- PMCID: PMC5312326
- DOI: 10.18632/oncotarget.10699
Short-term pyrrolidine dithiocarbamate administration attenuates cachexia-induced alterations to muscle and liver in ApcMin/+ mice
Abstract
Cancer cachexia is a complex wasting condition characterized by chronic inflammation, disrupted energy metabolism, and severe muscle wasting. While evidence in pre-clinical cancer cachexia models have determined that different systemic inflammatory inhibitors can attenuate several characteristics of cachexia, there is a limited understanding of their effects after cachexia has developed, and whether short-term administration is sufficient to reverse cachexia-induced signaling in distinctive target tissues. Pyrrolidine dithiocarbamate (PDTC) is a thiol compound having anti-inflammatory and antioxidant properties which can inhibit STAT3 and nuclear factor κB (NF-κB) signaling in mice. This study examined the effect of short-term PDTC administration to ApcMin/+ mice on cachexia-induced disruption of skeletal muscle protein turnover and liver metabolic function. At 16 weeks of age ApcMin/+ mice initiating cachexia (7% BW loss) were administered PDTC (10mg/kg bw/d) for 2 weeks. Control ApcMin/+ mice continued to lose body weight during the treatment period, while mice receiving PDTC had no further body weight decrease. PDTC had no effect on either intestinal tumor burden or circulating IL-6. In muscle, PDTC rescued signaling disrupting protein turnover regulation. PDTC suppressed the cachexia induction of STAT3, increased mTORC1 signaling and protein synthesis, and suppressed the induction of Atrogin-1 protein expression. Related to cachectic liver metabolic function, PDTC treatment attenuated glycogen and lipid content depletion independent to the activation of STAT3 and mTORC1 signaling. Overall, these results demonstrate short-term PDTC treatment to cachectic mice attenuated cancer-induced disruptions to muscle and liver signaling, and these changes were independent to altered tumor burden and circulating IL-6.
Keywords: hepatomegaly; inflammation; metabolism; muscle atrophy; protein turnover.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


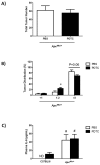




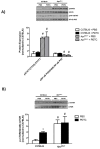

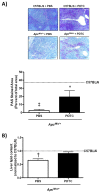
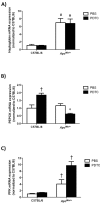
References
-
- Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. - PubMed
-
- Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2:862–871. - PubMed
-
- Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90–99. - PubMed
-
- Baracos VE. Clinical trials of cancer cachexia therapy, now and hereafter. J Clin Oncol. 2013;31:1257–1258. - PubMed
-
- Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

