Visual landmarks sharpen grid cell metric and confer context specificity to neurons of the medial entorhinal cortex
- PMID: 27449281
- PMCID: PMC4987135
- DOI: 10.7554/eLife.16937
Visual landmarks sharpen grid cell metric and confer context specificity to neurons of the medial entorhinal cortex
Abstract
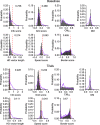
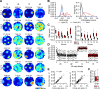
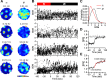
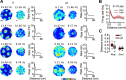
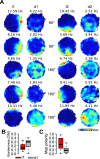
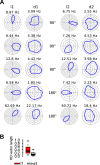
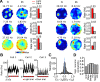
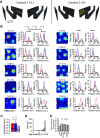
Neurons of the medial entorhinal cortex (MEC) provide spatial representations critical for navigation. In this network, the periodic firing fields of grid cells act as a metric element for position. The location of the grid firing fields depends on interactions between self-motion information, geometrical properties of the environment and nonmetric contextual cues. Here, we test whether visual information, including nonmetric contextual cues, also regulates the firing rate of MEC neurons. Removal of visual landmarks caused a profound impairment in grid cell periodicity. Moreover, the speed code of MEC neurons changed in darkness and the activity of border cells became less confined to environmental boundaries. Half of the MEC neurons changed their firing rate in darkness. Manipulations of nonmetric visual cues that left the boundaries of a 1D environment in place caused rate changes in grid cells. These findings reveal context specificity in the rate code of MEC neurons.
Keywords: border cells; grid cell; medial entorhinal cortex; mouse; navigation; neuroscience; path integration; speed cells.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


Similar articles
-
Absence of Visual Input Results in the Disruption of Grid Cell Firing in the Mouse.Curr Biol. 2016 Sep 12;26(17):2335-42. doi: 10.1016/j.cub.2016.06.043. Epub 2016 Aug 4. Curr Biol. 2016. PMID: 27498565 Free PMC article.
-
Environmental boundaries as a mechanism for correcting and anchoring spatial maps.J Physiol. 2016 Nov 15;594(22):6501-6511. doi: 10.1113/JP270624. Epub 2016 Jan 5. J Physiol. 2016. PMID: 26563618 Free PMC article. Review.
-
The Firing Rate Speed Code of Entorhinal Speed Cells Differs across Behaviorally Relevant Time Scales and Does Not Depend on Medial Septum Inputs.J Neurosci. 2019 May 1;39(18):3434-3453. doi: 10.1523/JNEUROSCI.1450-18.2019. Epub 2019 Feb 25. J Neurosci. 2019. PMID: 30804092 Free PMC article.
-
Visual cue-related activity of cells in the medial entorhinal cortex during navigation in virtual reality.Elife. 2020 Mar 9;9:e43140. doi: 10.7554/eLife.43140. Elife. 2020. PMID: 32149601 Free PMC article.
-
Ten Years of Grid Cells.Annu Rev Neurosci. 2016 Jul 8;39:19-40. doi: 10.1146/annurev-neuro-070815-013824. Epub 2016 Mar 9. Annu Rev Neurosci. 2016. PMID: 27023731 Review.
Cited by
-
During hippocampal inactivation, grid cells maintain synchrony, even when the grid pattern is lost.Elife. 2019 Oct 17;8:e47147. doi: 10.7554/eLife.47147. Elife. 2019. PMID: 31621577 Free PMC article.
-
Microcircuits for spatial coding in the medial entorhinal cortex.Physiol Rev. 2022 Apr 1;102(2):653-688. doi: 10.1152/physrev.00042.2020. Epub 2021 Jul 13. Physiol Rev. 2022. PMID: 34254836 Free PMC article. Review.
-
Place and Grid Cells in a Loop: Implications for Memory Function and Spatial Coding.J Neurosci. 2017 Aug 23;37(34):8062-8076. doi: 10.1523/JNEUROSCI.3490-16.2017. Epub 2017 Jul 12. J Neurosci. 2017. PMID: 28701481 Free PMC article.
-
A single-cell spiking model for the origin of grid-cell patterns.PLoS Comput Biol. 2017 Oct 2;13(10):e1005782. doi: 10.1371/journal.pcbi.1005782. eCollection 2017 Oct. PLoS Comput Biol. 2017. PMID: 28968386 Free PMC article.
-
Effects of visual inputs on neural dynamics for coding of location and running speed in medial entorhinal cortex.Elife. 2020 Dec 10;9:e62500. doi: 10.7554/eLife.62500. Elife. 2020. PMID: 33300873 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

