Ramosetron versus ondansetron for postoperative nausea and vomiting in strabismus surgery patients
- PMID: 27449404
- PMCID: PMC4957928
- DOI: 10.1186/s12871-016-0210-5
Ramosetron versus ondansetron for postoperative nausea and vomiting in strabismus surgery patients
Abstract
Background: Postoperative nausea and vomiting (PONV) is one of the most common adverse outcomes after strabismus surgery. The primary outcome of this prospective, randomized, double-blind study was to compare the incidences of nausea or vomiting, and patient satisfaction of ondansetron and ramosetron after strabismus surgery under general anesthesia. The secondary outcome was to investigate whether the number of involved extraocular muscles (EOMs) in strabismus surgery was related to PONV.
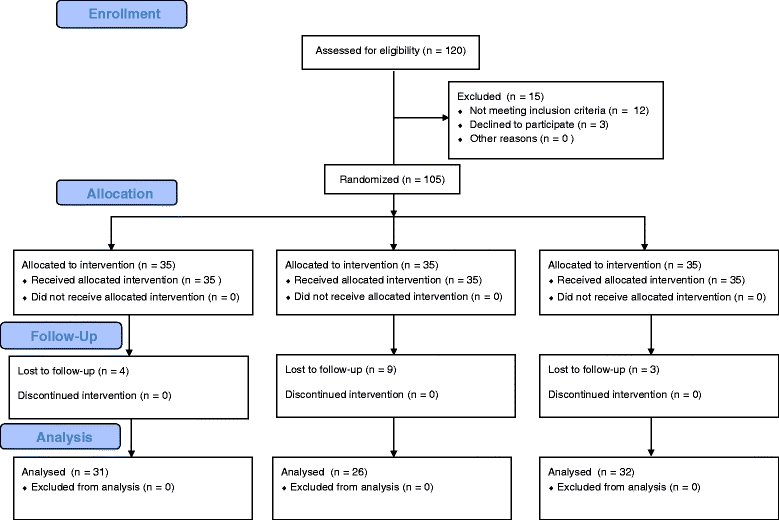
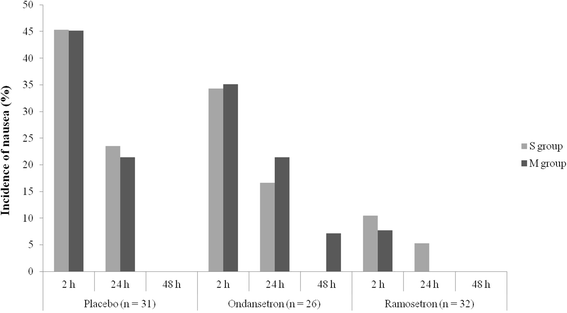
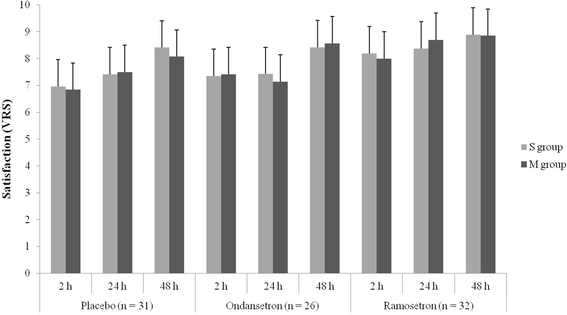
Methods: One hundred and five patients (aged 18-60 years) undergoing strabismus surgery were allocated randomly to one of the three groups: placebo, ondansetron, or ramosetron. Patients received 2 ml placebo, 4 mg ondansetron, or 0.3 mg ramosetron at the end of surgery. Each of the three groups was subdivided into two subgroups according to the number of EOMs involved in the surgery: subgroup S, single-muscle correction; subgroup M, multiple-muscle correction. The incidences of nausea or vomiting, and patient satisfaction at 2, 24 and 48 h after surgery were analyzed as primary outcome. With regard to subgroups S and M in the placebo, ondansetron and ramosetron groups, incidences of nausea or vomiting, and patient satisfaction at 2, 24 and 48 h after surgery were analyzed as seconadary outcome.
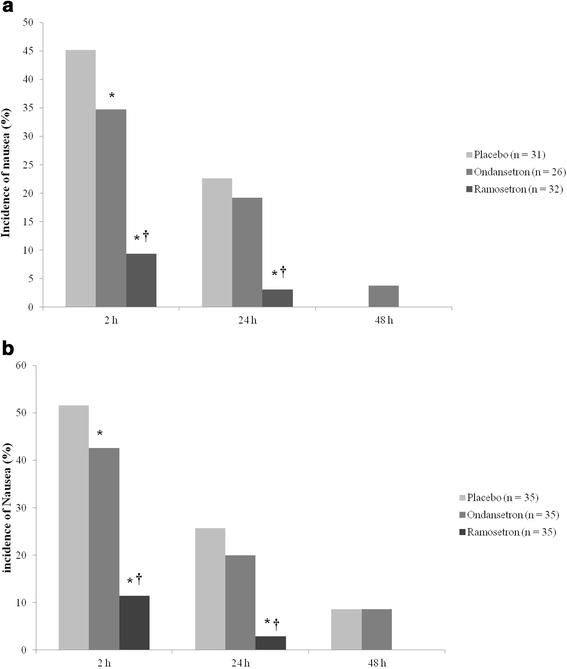
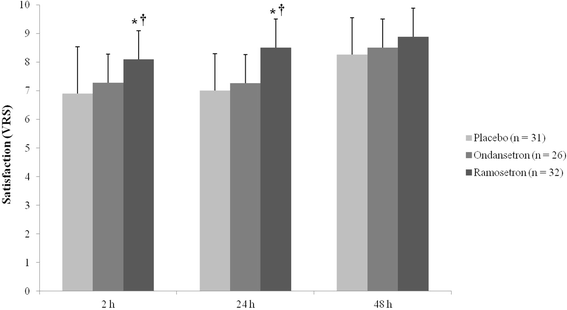
Results: The incidence of nausea was significantly lower in the ramosetron group at 2 h (9.4 %) than in the placebo (45.2 %) and ondansetron (34.7 %) groups (P < 0.05). The incidence of nausea was also significantly lower in the ramosetron group at 24 h than in the other groups (P < 0.05). Patients in the ramosetron group were more satisfied at 2 h (8.11 ± 0.98) and 24 h (8.50 ± 0.67) after surgery than those in the other groups (P < 0.05). With regard to subgroups S and M in the placebo, ondansetron and ramosetron groups, there were no significant differences in either the incidence of nausea or patient satisfaction.
Conclusion: Ramosetron has superior antiemetic activity to ondansetron in adult strabismus surgery patients. The number of EOMs involved in strabismus surgery was not related to the incidence of PONV.
Trial registration: Clinical Research Information Service (CRiS) Identifier: KCT0000688 . Date of registration: 27 February 2013.
Keywords: Ondansetron; Postoperative nausea; Ramosetron; Strabismus surgery.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

