Inadequate food and water intake determine mortality following stroke in mice
- PMID: 27449604
- PMCID: PMC5464703
- DOI: 10.1177/0271678X16660986
Inadequate food and water intake determine mortality following stroke in mice
Abstract
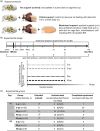
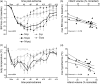
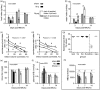
Experimental stroke models producing clinically relevant functional deficits are often associated with high mortality. Because the mechanisms that underlie post-stroke mortality are largely unknown, results obtained using these models are often difficult to interpret, thereby limiting their translational potential. Given that specific forms of post-stroke care reduce mortality in patients, we hypothesized that inadequate food and water intake may underlie mortality following experimental stroke. C57BL/6 mice were subjected to 1 h of intraluminal filament middle cerebral artery occlusion. Nutritional support beginning on the second day after filament middle cerebral artery occlusion reduced the 14-day mortality rate from 59% to 15%. The surviving mice in the post-stroke support group had the same infarct size as non-surviving control mice, suggesting that post-stroke care was not neuroprotective and that inadequate food and/or water intake are the main reasons for filament middle cerebral artery occlusion-induced mortality. This notion was supported by the presence of significant hypoglycemia, ketonemia, and dehydration in control mice. Taken together, these data suggest that post-filament middle cerebral artery occlusion mortality in mice is not primarily caused by ischemic brain damage, but secondarily by inadequate food and/or water intake. Thus, providing nutritional support following filament middle cerebral artery occlusion greatly minimizes mortality bias and allows the study of long-term morphological and functional sequelae of stroke in mice.
Keywords: chronic stroke model; filament middle cerebral artery occlusion; mortality; stroke care; translational medicine.
Figures



References
-
- Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet 2008; 371: 1612–1623. - PubMed
-
- Grotta JC, Hacke W. Stroke neurologist's perspective on the new endovascular trials. Stroke 2015; 46: 1447–1452. - PubMed
-
- Dirnagl U, Endres M. Found in translation: preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke 2014; 45: 1510–1518. - PubMed
-
- O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol 2006; 59: 467–477. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

