Evaluating the efficacy of dexamethasone in the treatment of patients with persistent acute respiratory distress syndrome: study protocol for a randomized controlled trial
- PMID: 27449641
- PMCID: PMC4957909
- DOI: 10.1186/s13063-016-1456-4
Evaluating the efficacy of dexamethasone in the treatment of patients with persistent acute respiratory distress syndrome: study protocol for a randomized controlled trial
Abstract
Background: Although much has evolved in our understanding of the pathogenesis and factors affecting outcome of patients with acute respiratory distress syndrome (ARDS), still there is no specific pharmacologic treatment for ARDS. Several clinical trials have evaluated the utility of corticoids but none of them has demonstrated a definitive benefit due to small sample sizes, selection bias, patient heterogeneity, and time of initiation of treatment or duration of therapy. We postulated that adjunctive treatment of persistent ARDS with intravenous dexamethasone might change the pulmonary and systemic inflammatory response and thereby reduce morbidity, leading to a decrease in duration of mechanical ventilation and a decrease in mortality.
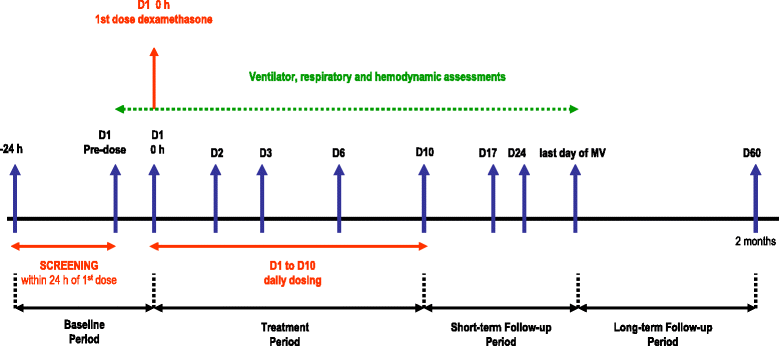
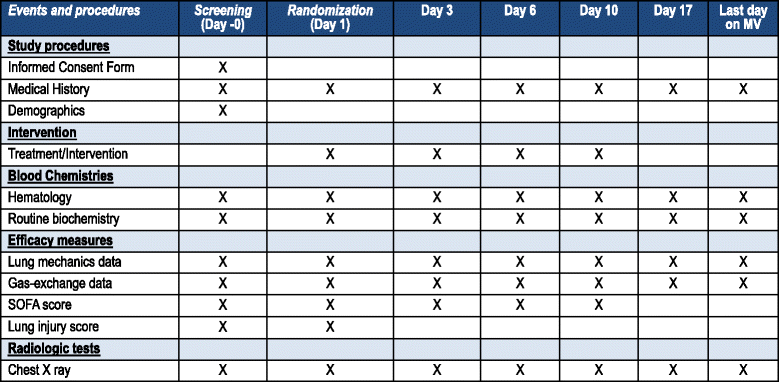
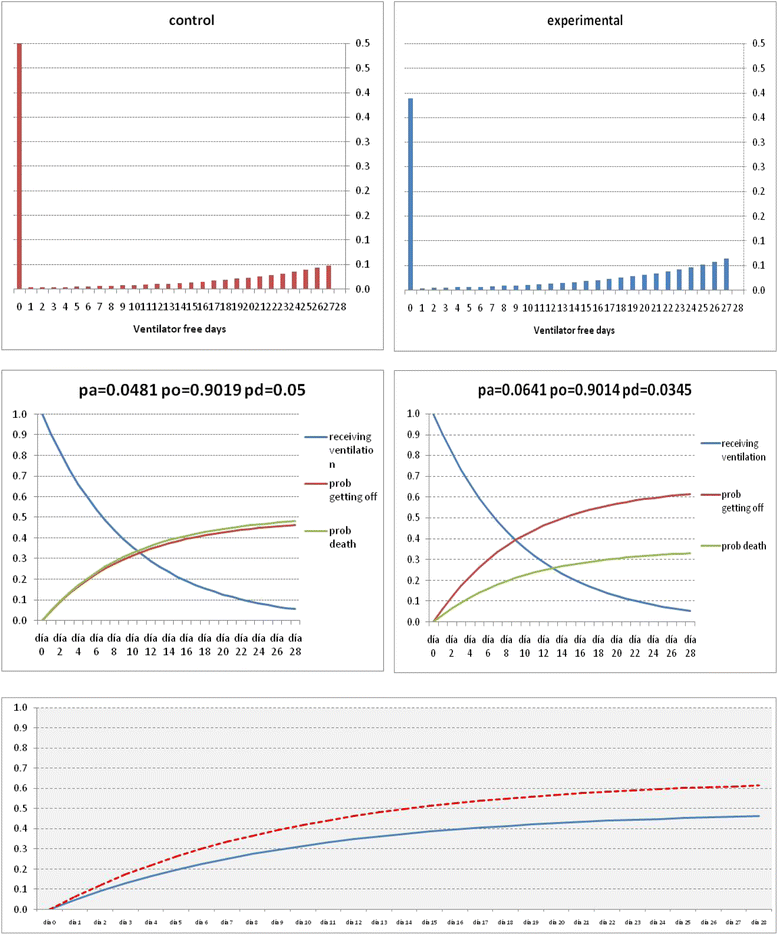
Methods/design: This is a prospective, multicenter, randomized, controlled trial in 314 patients with persistent moderate/severe ARDS. Persistent ARDS is defined as maintaining a PaO2/FiO2 ≤ 200 mmHg on PEEP ≥ 10 cmH2O and FiO2 ≥ 0.5 after 24 hours of routine intensive care. Eligible patients will be randomly allocated to two arms: (i) conventional treatment without dexamethasone, (ii) conventional treatment plus dexamethasone. Patients in the dexamethasone group will be treated with a daily dose of 20 mg iv from day 1 to day 5, and 10 mg iv from day 6 to day 10. Primary outcome is the number of ventilator-free days, defined as days alive and free from mechanical ventilation at day 28 after intubation. Secondary outcome is all-cause mortality at day 60 after enrollment.
Discussion: This study will be the largest randomized controlled clinical trial to assess the role of dexamethasone in patients with persistent ARDS.
Trial registration: Registered on 21 November 2012 as DEXA-ARDS at ClinicalTrials.gov website ( NCT01731795 ).
Keywords: Acute respiratory distress syndrome; Corticoids; Dexamethasone; Lung-protective ventilation; Positive end-expiratory pressure.
Figures
References
-
- Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa S, Fernández RL, Kacmarek RM. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;7:1932–41. - PubMed
-
- Monton C, Ewig S, Torres A, El-Ebiary M, Filella X, Rañó A, Xaubet A. Role of glucocorticoids on inflammatory response in nonimmunosupressed patients with pneumonia: a pilot study. Eur Respir J. 1999;14:218–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

