Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia
- PMID: 27449665
- PMCID: PMC4957923
- DOI: 10.1186/s12889-016-3297-1
Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia
Abstract
Background: Annual trivalent influenza vaccines (TIV) containing three influenza strains (A/H1N1, A/H3N2, and one B) have been recommended for the prevention of influenza. However, worldwide co-circulation of two distinct B lineages (Victoria and Yamagata) and difficulties in predicting which lineage will predominate each season have led to the development of quadrivalent influenza vaccines (QIV), which include both B lineages. Our analysis evaluates the public health benefit and associated influenza-related costs avoided which would have been obtained by using QIV rather than TIV in Australia over the period 2002-2012.
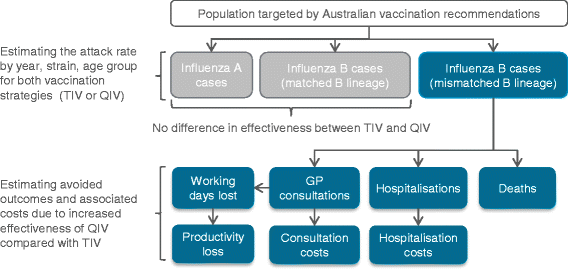
Methods: A static model stratified by age group was used, focusing on people at increased risk of influenza as defined by the Australian vaccination recommendations. B-lineage cross-protection was accounted for. We calculated the potential impact of QIV compared with TIV over the seasons 2002-2012 (2009 pandemic year excluded) using Australian data on influenza circulation, vaccine coverage, hospitalisation and mortality rates as well as unit costs, and international data on vaccine effectiveness, influenza attack rate, GP consultation rate and working days lost. Third-party payer and societal influenza-related costs were estimated in 2014 Australian dollars. Sensitivity analyses were conducted.
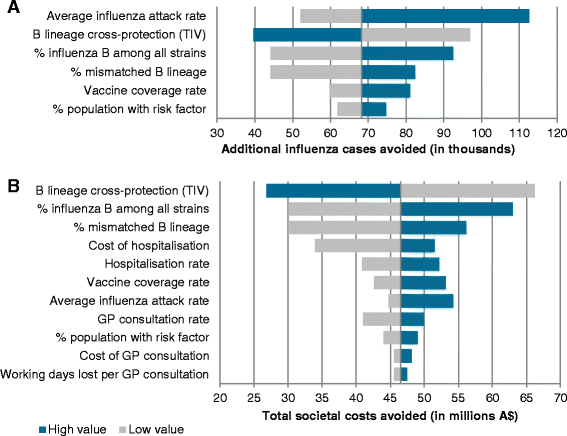
Results: Using QIV instead of TIV over the period 2002-2012 would have prevented an estimated 68,271 additional influenza cases, 47,537 GP consultations, 3,522 hospitalisations and 683 deaths in the population at risk of influenza. These results translate into influenza-related societal costs avoided of $46.5 million. The estimated impact of QIV was higher for young children and the elderly. The overall impact of QIV depended mainly on vaccine effectiveness and the influenza attack rate attributable to the mismatched B lineage.
Conclusion: The broader protection offered by QIV would have reduced the number of influenza infections and its related complications, leading to substantial influenza-related costs avoided.
Keywords: Australia; Benefit; Cost; Influenza; Public health; Quadrivalent; Vaccine.
Figures
Similar articles
-
Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine.Vaccine. 2012 Mar 2;30(11):1993-8. doi: 10.1016/j.vaccine.2011.12.098. Epub 2012 Jan 5. Vaccine. 2012. PMID: 22226861
-
Public health impact and economic benefits of quadrivalent influenza vaccine in Latin America.Hum Vaccin Immunother. 2017 Apr 3;13(4):877-888. doi: 10.1080/21645515.2016.1256928. Epub 2017 Jan 24. Hum Vaccin Immunother. 2017. PMID: 28118092 Free PMC article.
-
Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years.BMC Infect Dis. 2013 Jul 24;13:343. doi: 10.1186/1471-2334-13-343. BMC Infect Dis. 2013. PMID: 23883186 Free PMC article. Clinical Trial.
-
Comparison of the immunogenicity and safety of quadrivalent and tetravalent influenza vaccines in children and adolescents.Vaccine. 2020 Feb 5;38(6):1332-1344. doi: 10.1016/j.vaccine.2019.11.071. Epub 2020 Jan 14. Vaccine. 2020. PMID: 31948819 Review.
-
[Immunogenicity of inacitivated quadrivalent influenza vaccine in adults aged 18-64 years: A systematic review and Meta-analysis].Zhonghua Liu Xing Bing Xue Za Zhi. 2018 Dec 10;39(12):1636-1641. doi: 10.3760/cma.j.issn.0254-6450.2018.12.019. Zhonghua Liu Xing Bing Xue Za Zhi. 2018. PMID: 30572392 Chinese.
Cited by
-
The Epidemiology and Burden of Influenza B/Victoria and B/Yamagata Lineages in Kenya, 2012-2016.Open Forum Infect Dis. 2019 Sep 30;6(10):ofz421. doi: 10.1093/ofid/ofz421. eCollection 2019 Oct. Open Forum Infect Dis. 2019. PMID: 31660376 Free PMC article.
-
Molecular evolution of influenza B virus during 2011-2017 in Chaoyang, Beijing, suggesting the free influenza vaccine policy.Sci Rep. 2019 Feb 21;9(1):2432. doi: 10.1038/s41598-018-38105-1. Sci Rep. 2019. PMID: 30792414 Free PMC article.
-
mRNA-based COVID-19 booster vaccination is highly effective and cost-effective in Australia.Vaccine. 2023 Apr 6;41(15):2439-2446. doi: 10.1016/j.vaccine.2023.01.075. Epub 2023 Feb 3. Vaccine. 2023. PMID: 36781332 Free PMC article.
-
Vaccine Mismatches, Viral Circulation, and Clinical Severity Patterns of Influenza B Victoria and Yamagata Infections in Brazil over the Decade 2010-2020: A Statistical and Phylogeny-Trait Analyses.Viruses. 2022 Jul 5;14(7):1477. doi: 10.3390/v14071477. Viruses. 2022. PMID: 35891457 Free PMC article.
-
Estimated cost-effectiveness and burden of disease associated with quadrivalent cell-based and egg-based influenza vaccines in Spain.Hum Vaccin Immunother. 2020 Sep 1;16(9):2238-2244. doi: 10.1080/21645515.2020.1712935. Epub 2020 Feb 10. Hum Vaccin Immunother. 2020. PMID: 32040379 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC). People at High Risk of Developing Flu–Related Complications. http://www.cdc.gov/flu/about/disease/high_risk.htm. Accessed 01 Mar 2016.
-
- Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2004;53:1–40. - PubMed
-
- World Health Organization (WHO). Influenza Fact sheet n°211. 2014. http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed 01 Mar 2016.
-
- Centers for Disease Control and Prevention (CDC). Vaccine Effectiveness - How well does the flu vaccine work ? http://www.cdc.gov/flu/about/qa/vaccineeffect.htm. Accessed 01 Mar 2016.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

