The complexity of small circuits: the stomatogastric nervous system
- PMID: 27450880
- PMCID: PMC5270626
- DOI: 10.1016/j.conb.2016.07.005
The complexity of small circuits: the stomatogastric nervous system
Abstract
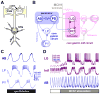
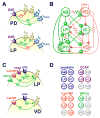
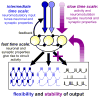
The crustacean stomatogastric nervous system is a long-standing test bed for studies of circuit dynamics and neuromodulation. We give a brief update on the most recent work on this system, with an emphasis on the broader implications for understanding neural circuits. In particular, we focus on new findings underlining that different levels of dynamics taking place at different time scales all interact in multiple ways. Dynamics due to synaptic and intrinsic neuronal properties, neuromodulation, and long-term gene expression-dependent regulation are not independent, but influence each other. Extensive research on the stomatogastric system shows that these dynamic interactions convey robustness to circuit operation, while facilitating the flexibility of producing multiple circuit outputs.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
statement Nothing declared.
Figures
Similar articles
-
The roles of co-transmission in neural network modulation.Trends Neurosci. 2001 Mar;24(3):146-54. doi: 10.1016/s0166-2236(00)01723-9. Trends Neurosci. 2001. PMID: 11182454 Review.
-
Identifiable cells in the crustacean stomatogastric ganglion.Physiology (Bethesda). 2010 Oct;25(5):311-8. doi: 10.1152/physiol.00019.2010. Physiology (Bethesda). 2010. PMID: 20940436 Review.
-
Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs.Annu Rev Physiol. 2007;69:291-316. doi: 10.1146/annurev.physiol.69.031905.161516. Annu Rev Physiol. 2007. PMID: 17009928 Review.
-
A small-systems approach to motor pattern generation.Nature. 2002 May 16;417(6886):343-50. doi: 10.1038/417343a. Nature. 2002. PMID: 12015615 Free PMC article. Review.
-
Neural architecture: from cells to circuits.J Neurophysiol. 2018 Aug 1;120(2):854-866. doi: 10.1152/jn.00044.2018. Epub 2018 May 16. J Neurophysiol. 2018. PMID: 29766767 Free PMC article. Review.
Cited by
-
Combining Old and New Tricks: The Study of Genes, Neurons, and Behavior in Crayfish.Front Physiol. 2022 Jul 6;13:947598. doi: 10.3389/fphys.2022.947598. eCollection 2022. Front Physiol. 2022. PMID: 35874546 Free PMC article.
-
A perspective on neuroethology: what the past teaches us about the future of neuroethology.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Mar;210(2):325-346. doi: 10.1007/s00359-024-01695-5. Epub 2024 Feb 27. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 38411712 Free PMC article. Review.
-
Dopamine maintains network synchrony via direct modulation of gap junctions in the crustacean cardiac ganglion.Elife. 2018 Oct 16;7:e39368. doi: 10.7554/eLife.39368. Elife. 2018. PMID: 30325308 Free PMC article.
-
SIFamide peptides modulate cardiac activity differently in two species of Cancer crab.Gen Comp Endocrinol. 2019 Oct 1;282:113204. doi: 10.1016/j.ygcen.2019.06.008. Epub 2019 Jun 12. Gen Comp Endocrinol. 2019. PMID: 31201801 Free PMC article.
-
The Gastric Ganglion of Octopus vulgaris: Preliminary Characterization of Gene- and Putative Neurochemical-Complexity, and the Effect of Aggregata octopiana Digestive Tract Infection on Gene Expression.Front Physiol. 2017 Dec 15;8:1001. doi: 10.3389/fphys.2017.01001. eCollection 2017. Front Physiol. 2017. PMID: 29326594 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

