Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial
- PMID: 27451390
- PMCID: PMC5541855
- DOI: 10.1016/S1470-2045(16)30167-X
Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial
Abstract
Background: Malignant cells of classical Hodgkin's lymphoma are characterised by genetic alterations at the 9p24.1 locus, leading to overexpression of PD-1 ligands and evasion of immune surveillance. In a phase 1b study, nivolumab, a PD-1-blocking antibody, produced a high response in patients with relapsed and refractory classical Hodgkin's lymphoma, with an acceptable safety profile. We aimed to assess the clinical benefit and safety of nivolumab monotherapy in patients with classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin.
Methods: In this ongoing, single-arm phase 2 study, adult patients (aged ≥18 years) with recurrent classical Hodgkin's lymphoma who had failed to respond to autologous stem-cell transplantation and had either relapsed after or failed to respond to brentuximab vedotin, and with an Eastern Cooperative Oncology Group performance status score of 0 or 1, were enrolled from 34 hospitals and academic centres across Europe and North America. Patients were given nivolumab intravenously over 60 min at 3 mg/kg every 2 weeks until progression, death, unacceptable toxicity, or withdrawal from study. The primary endpoint was objective response following a prespecified minimum follow-up period of 6 months, assessed by an independent radiological review committee (IRRC). All patients who received at least one dose of nivolumab were included in the primary and safety analyses. This trial is registered with ClinicalTrials.gov, number NCT02181738.
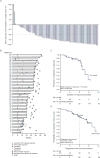
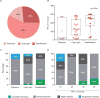
Findings: Among 80 treated patients recruited between Aug 26, 2014, and Feb 20, 2015, the median number of previous therapies was four (IQR 4-7). At a median follow-up of 8·9 months (IQR 7·8-9·9), 53 (66·3%, 95% CI 54·8-76·4) of 80 patients achieved an IRRC-assessed objective response. The most common drug-related adverse events (those that occurred in ≥15% of patients) included fatigue (20 [25%] patients), infusion-related reaction (16 [20%]), and rash (13 [16%]). The most common drug-related grade 3 or 4 adverse events were neutropenia (four [5%] patients) and increased lipase concentrations (four [5%]). The most common serious adverse event (any grade) was pyrexia (three [4%] patients). Three patients died during the study; none of these deaths were judged to be treatment related.
Interpretation: Nivolumab resulted in frequent responses with an acceptable safety profile in patients with classical Hodgkin's lymphoma who progressed after autologous stem-cell transplantation and brentuximab vedotin. Therefore, nivolumab might be a new treatment option for a patient population with a high unmet need. Ongoing follow-up will help to assess the durability of response.
Funding: Bristol-Myers Squibb.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
AS, PLZ, GC, MR, and AHL declare no competing interests.
Figures
Comment in
-
Is nivolumab an option for Hodgkin's lymphoma?Lancet Oncol. 2016 Sep;17(9):1183-4. doi: 10.1016/S1470-2045(16)30220-0. Epub 2016 Jul 20. Lancet Oncol. 2016. PMID: 27451389 No abstract available.
References
-
- Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–71. - PubMed
-
- Martinez C, Canals C, Sarina B, et al. Identification of prognostic factors predicting outcome in Hodgkin’s lymphoma patients relapsing after autologous stem cell transplantation. Ann Oncol. 2013;24:2430–4. - PubMed
-
- Arai S, Fanale M, DeVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54:2531–3. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

