Short-term outcomes of minimally invasive versus open colectomy for colon cancer
- PMID: 27451872
- PMCID: PMC6475900
- DOI: 10.1016/j.jss.2016.04.020
Short-term outcomes of minimally invasive versus open colectomy for colon cancer
Abstract
Background: Laparoscopic and open approaches to colon resection have equivalent long-term outcomes and oncologic integrity for the treatment of colon cancer. Differences in short-term outcomes should therefore help to guide surgeons in their choice of operation. We hypothesized that minimally invasive colectomy is associated with superior short-term outcomes compared to traditional open colectomy in the setting of colon cancer.
Materials and methods: Patients undergoing nonemergent colectomy for colon cancer in 2012 and 2013 were selected from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) targeted colectomy participant use file. Patients were divided into two cohorts based on operative approach-open versus minimally invasive surgery (MIS). Univariate, multivariate, and propensity-adjusted multivariate analyses were performed to compare postoperative outcomes between the two groups.
Results: A total of 11,031 patients were identified for inclusion in the study, with an overall MIS rate of 65.3% (n = 7200). On both univariate and multivariate analysis, MIS approach was associated with fewer postoperative complications and lower mortality. In the risk-adjusted multivariate analysis, MIS approach was associated with an odds ratio of 0.598 for any postoperative morbidity compared to open (P < 0.001).
Conclusions: This retrospective study of patients undergoing colectomy for colon cancer demonstrates significantly improved outcomes associated with a MIS approach, even when controlling for baseline differences in illness severity. When feasible, minimally invasive colectomy should be considered gold standard for the surgical treatment of colon cancer.
Keywords: Colectomy; Colonic neoplasms; Laparoscopy; Minimally invasive surgical procedures.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

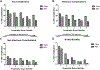


References
-
- Monson JR, Darzi A, Carey PD, Guillou PJ. Prospective evaluation of laparoscopic-assisted colectomy in an unselected group of patients. Lancet 1992;340:831–833. - PubMed
-
- Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991;1:144–150. - PubMed
-
- Berends FJ, Kazemier G, Bonjer HJ, Lange JF. Subcutaneous metastases after laparoscopic colectomy. Lancet 1994; 344:58. - PubMed
-
- Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the Uk MRC CLASICC Trial Group. J Clin Oncol 2007;25:3061–3068. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

