Efficacy and safety of tofacitinib in patients with active rheumatoid arthritis: review of key Phase 2 studies
- PMID: 27451980
- PMCID: PMC5396326
- DOI: 10.1111/1756-185X.12901
Efficacy and safety of tofacitinib in patients with active rheumatoid arthritis: review of key Phase 2 studies
Abstract
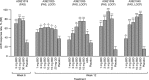
Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). Here, the safety and efficacy data from five Phase 2 studies of tofacitinib in patients with RA are summarized. Tofacitinib 1-30 mg twice daily was investigated, as monotherapy and in combination with methotrexate, in patients with RA. Tofacitinib 20 mg once daily was investigated in one study. Tofacitinib 5 and 10 mg twice daily were selected for investigation in Phase 3 studies; therefore, the efficacy and safety of tofacitinib 5 and 10 mg twice daily in Phase 2 studies are the focus of this review. Tofacitinib ≥ 5 mg twice daily was efficacious in a dose-dependent manner, with statistically significant and clinically meaningful reductions in the signs and symptoms of RA and patient-reported outcomes. The safety profile was consistent across studies. The efficacy and safety profile of tofacitinib in Phase 2 studies supported its further investigation and the selection of tofacitinib 5 mg twice daily and tofacitinib 10 mg twice daily for evaluation in Phase 3 studies.
Keywords: Phase 2; efficacy; rheumatoid arthritis; safety; tofacitinib.
© 2016 The Authors. International Journal of Rheumatic Diseases published by Asia Pacific League of Associations for Rheumatology and John Wiley & Sons Australia, Ltd.
Figures
References
-
- Strand V, Khanna D (2010) The impact of rheumatoid arthritis and treatment on patients’ lives. Clin Exp Rheumatol 28, S32–40. - PubMed
-
- Rezaei H, Saevarsdottir S, Forslind K et al (2012) In early rheumatoid arthritis, patients with a good initial response to methotrexate have excellent 2‐year clinical outcomes, but radiological progression is not fully prevented: data from the methotrexate responders population in the SWEFOT trial. Ann Rheum Dis 71, 186–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

