A Salmonella nanoparticle mimic overcomes multidrug resistance in tumours
- PMID: 27452236
- PMCID: PMC5512628
- DOI: 10.1038/ncomms12225
A Salmonella nanoparticle mimic overcomes multidrug resistance in tumours
Abstract
Salmonella enterica serotype Typhimurium is a food-borne pathogen that also selectively grows in tumours and functionally decreases P-glycoprotein (P-gp), a multidrug resistance transporter. Here we report that the Salmonella type III secretion effector, SipA, is responsible for P-gp modulation through a pathway involving caspase-3. Mimicking the ability of Salmonella to reverse multidrug resistance, we constructed a gold nanoparticle system packaged with a SipA corona, and found this bacterial mimic not only accumulates in tumours but also reduces P-gp at a SipA dose significantly lower than free SipA. Moreover, the Salmonella nanoparticle mimic suppresses tumour growth with a concomitant reduction in P-gp when used with an existing chemotherapeutic drug (that is, doxorubicin). On the basis of our finding that the SipA Salmonella effector is fundamental for functionally decreasing P-gp, we engineered a nanoparticle mimic that both overcomes multidrug resistance in cancer cells and increases tumour sensitivity to conventional chemotherapeutics.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
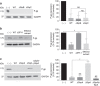

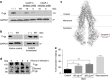


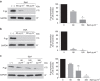
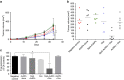
Comment in
-
Biomimetic Salmonella: A Next-Generation Therapeutic Vector?Trends Microbiol. 2016 Nov;24(11):850-852. doi: 10.1016/j.tim.2016.08.007. Epub 2016 Sep 7. Trends Microbiol. 2016. PMID: 27614692
References
-
- Pawelek J. M., Low K. B. & Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer. Res. 57, 4537–4544 (1997). - PubMed
-
- Low K. B. et al. Construction of VNP20009: a novel, genetically stable antibiotic-sensitive strain of tumor-targeting Salmonella for parenteral administration in humans. Methods Mol. Med. 90, 47–60 (2004). - PubMed
-
- Galan J. E. Molecular and cellular bases of Salmonella entry into host cells. Curr. Top. Microbiol. Immunol. 209, 43–60 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

