ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs
- PMID: 27452466
- PMCID: PMC4972690
- DOI: 10.1016/j.celrep.2016.06.081
ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs
Abstract
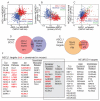
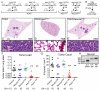
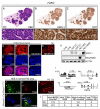
Small cell lung carcinoma (SCLC) is a high-grade pulmonary neuroendocrine tumor. The transcription factors ASCL1 and NEUROD1 play crucial roles in promoting malignant behavior and survival of human SCLC cell lines. Here, we find that ASCL1 and NEUROD1 identify heterogeneity in SCLC, bind distinct genomic loci, and regulate mostly distinct genes. ASCL1, but not NEUROD1, is present in mouse pulmonary neuroendocrine cells, and only ASCL1 is required in vivo for tumor formation in mouse models of SCLC. ASCL1 targets oncogenic genes including MYCL1, RET, SOX2, and NFIB while NEUROD1 targets MYC. ASCL1 and NEUROD1 regulate different genes that commonly contribute to neuronal function. ASCL1 also regulates multiple genes in the NOTCH pathway including DLL3. Together, ASCL1 and NEUROD1 distinguish heterogeneity in SCLC with distinct genomic landscapes and distinct gene expression programs.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. - PubMed
-
- Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, Baylin SB, Ball DW. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386:852–855. - PubMed
-
- Bunt J, Hasselt NE, Zwijnenburg DA, Hamdi M, Koster J, Versteeg R, Kool M. OTX2 directly activates cell cycle genes and inhibits differentiation in medulloblastoma cells. International journal of cancer Journal international du cancer. 2012;131:E21–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

