Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: example lists and criteria for their selection and use
- PMID: 27452664
- PMCID: PMC5250586
- DOI: 10.14573/altex.1604201
Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: example lists and criteria for their selection and use
Abstract
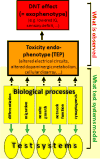
There is a paucity of information concerning the developmental neurotoxicity (DNT) hazard posed by industrial and environmental chemicals. New testing approaches will most likely be based on batteries of alternative and complementary (non-animal) tests. As DNT is assumed to result from the modulation of fundamental neurodevelopmental processes (such as neuronal differentiation, precursor cell migration or neuronal network formation) by chemicals, the first generation of alternative DNT tests target these processes. The advantage of such types of assays is that they capture toxicants with multiple targets and modes-of-action. Moreover, the processes modelled by the assays can be linked to toxicity endophenotypes, i.e., alterations in neural connectivity that form the basis for neurofunctional deficits in man. The authors of this review convened in a workshop to define criteria for the selection of positive/negative controls, to prepare recommendations on their use, and to initiate the setup of a directory of reference chemicals. For initial technical optimization of tests, a set of > 50 endpoint-specific control compounds was identified. For further test development, an additional "test" set of 33 chemicals considered to act directly as bona fide DNT toxicants is proposed, and each chemical is annotated to the extent it fulfills these criteria. A tabular compilation of the original literature used to select the test set chemicals provides information on statistical procedures, and toxic/non-toxic doses (both for pups and dams). Suggestions are provided on how to use the > 100 compounds (including negative controls) compiled here to address specificity, adversity and use of alternative test systems.
Keywords: AOP; neurotoxicity; specificity; test development; validation.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Albright TD, Jessell TM, Kandel ER, et al. Neural science: a century of progress and the mysteries that remain. Neuron. 2000;25(Suppl):S1–55. http://dx.doi.org/10.1016/s0896-6273(00)80912-5. - DOI - PubMed
-
- Alepee N, Bahinski A, Daneshian M, et al. State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology. ALTEX. 2014;31:441–477. http://dx.doi.org/http://dx.doi.org/10.14573/altex1406111. - DOI - PMC - PubMed
-
- Ali MM, Murthy RC, Chandra SV. Developmental and longterm neurobehavioral toxicity of low level in-utero cadmium exposure in rats. Neurobehav Toxicol Teratol. 1986;8:463–468. - PubMed
-
- Ankley GT, Bennett RS, Erickson RJ, et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29:730–741. http://dx.doi.org/10.1002/etc.34. - DOI - PubMed
-
- Asimiadou S, Bittigau P, Felderhoff-Mueser U, et al. Protection with estradiol in developmental models of apoptotic neurodegeneration. Ann Neurol. 2005;58:266–276. http://dx.doi.org/10.1002/ana.20553. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

