Multiple Origins of Virus Persistence during Natural Control of HIV Infection
- PMID: 27453467
- PMCID: PMC4983216
- DOI: 10.1016/j.cell.2016.06.039
Multiple Origins of Virus Persistence during Natural Control of HIV Infection
Abstract
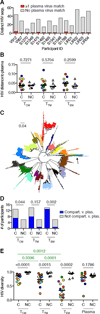
Targeted HIV cure strategies require definition of the mechanisms that maintain the virus. Here, we tracked HIV replication and the persistence of infected CD4 T cells in individuals with natural virologic control by sequencing viruses, T cell receptor genes, HIV integration sites, and cellular transcriptomes. Our results revealed three mechanisms of HIV persistence operating within distinct anatomic and functional compartments. In lymph node, we detected viruses with genetic and transcriptional attributes of active replication in both T follicular helper (TFH) cells and non-TFH memory cells. In blood, we detected inducible proviruses of archival origin among highly differentiated, clonally expanded cells. Linking the lymph node and blood was a small population of circulating cells harboring inducible proviruses of recent origin. Thus, HIV replication in lymphoid tissue, clonal expansion of infected cells, and recirculation of recently infected cells act together to maintain the virus in HIV controllers despite effective antiviral immunity.
Published by Elsevier Inc.
Figures






Comment in
-
HIV Reservoir: Finding the Right Needles in a Needlestack.Cell Host Microbe. 2016 Sep 14;20(3):280-282. doi: 10.1016/j.chom.2016.08.011. Cell Host Microbe. 2016. PMID: 27631698
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

