Nonoxidative removal of organics in the activated sludge process
- PMID: 27453679
- PMCID: PMC4940897
- DOI: 10.1080/10643389.2016.1149903
Nonoxidative removal of organics in the activated sludge process
Abstract
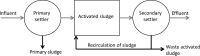
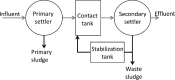
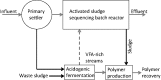
The activated sludge process is commonly used to treat wastewater by aerobic oxidation of organic pollutants into carbon dioxide and water. However, several nonoxidative mechanisms can also contribute to removal of organics. Sorption onto activated sludge can remove a large fraction of the colloidal and particulate wastewater organics. Intracellular storage of, e.g., polyhydroxyalkanoates (PHA), triacylglycerides (TAG), or wax esters can convert wastewater organics into precursors for high-value products. Recently, several environmental, economic, and technological drivers have stimulated research on nonoxidative removal of organics for wastewater treatment. In this paper, we review these nonoxidative removal mechanisms as well as the existing and emerging process configurations that make use of them for wastewater treatment. Better utilization of nonoxidative processes in activated sludge could reduce the wasteful aerobic oxidation of organic compounds and lead to more resource-efficient wastewater treatment plants.
Keywords: Adsorption; DLVO theory; colloids; contact-stabilization; high-rate activated sludge; polyhydroxyalkanoate; triacylglyceride.
Figures





References
-
- Akanyeti I., Temmink H., Remy M., Zwijnenburg A. Feasibility of bioflocculation in a high-loaded membrane bioreactor for improved energy recovery from sewage. Water Science & Technology. 2010;61:1433–1439. - PubMed
-
- Albuquerque M. G. E., Concas S., Bengtsson S., Reis M. A. M. Mixed culture polyhydroxyalkanoates production from sugar molasses: The use of a 2-stage CSTR system for culture selection. Bioresource Technology. 2010a;101:7112–7122. - PubMed
-
- Albuquerque M. G., Torres C. A., Reis M. A. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: Effect of the influent substrate concentration on culture selection. Water Research. 2010b;44(11):3419–3433. - PubMed
-
- Alexander W. V., Ekama G. A., Marais G. v. R. The activated sludge process part 2. Application of the general kinetic model to the contact stabilization process. Water Research. 1980;14:1737–1747.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
