Combinatorial epigenetic therapy in diffuse large B cell lymphoma pre-clinical models and patients
- PMID: 27453763
- PMCID: PMC4957280
- DOI: 10.1186/s13148-016-0245-y
Combinatorial epigenetic therapy in diffuse large B cell lymphoma pre-clinical models and patients
Abstract
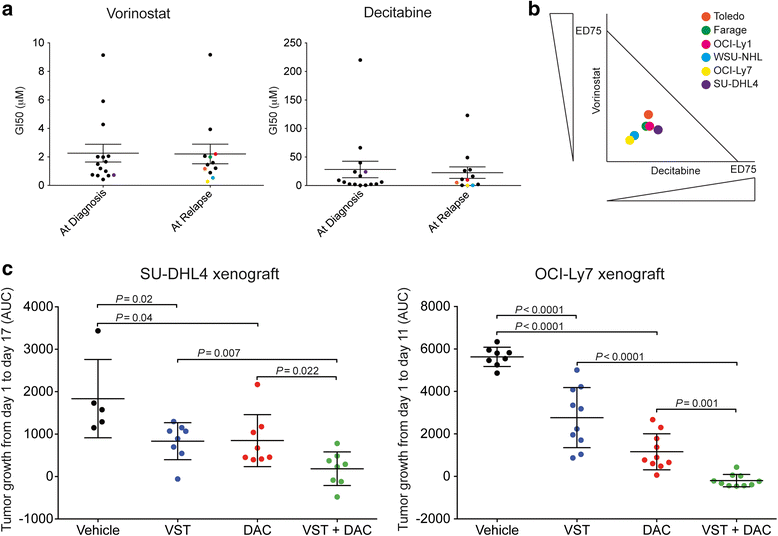
Background: Refractory and/or relapsed diffuse large B cell lymphoma (RR-DLBCL) patients are incurable with conventional chemotherapy due to the aggressiveness and the chemorefractory state of these tumors. DNA hypermethylation and histone deacetylation are two major epigenetic modifications by which aggressive DLBCL maintain their oncogenic state. We have previously reported that DNA methyltransferase inhibitors (DNMTI) affect RR-DLBCL growth and improve chemosensitivity. Here, we hypothesized that the combination of DNMTI with histone deacetylase inhibitor (HDI) would be an active and feasible therapeutic strategy in RR-DLBCL. Thus, we evaluated the anti-lymphoma activity of the HDI vorinostat (VST) in combination with the DNMTI azacitidine (AZA) or decitabine (DAC) in pre-clinical models of RR-DLBCL, and we determined the feasibility of the combination by conducting a phase Ib trial in RR-DLBCL patients.
Results: Concurrent combination of DNMTI and HDI resulted in synergistic anti-lymphoma effect toward RR-DLBCL cells in vitro and in vivo, with no significant toxicity increase. In a phase Ib trial, a total of 18 patients with a median of three prior therapies were treated with four different dose levels of AZA and VST. The most common toxicities were hematological, followed by gastrointestinal and metabolic. The clinical benefit was low as only one subject had a partial response and three subjects had stable disease. Interestingly, two of the seven patients that received additional chemotherapy post-study achieved a complete response and three others had a significant clinical benefit. These observations suggested that the combination might have a delayed chemosensitization effect that we were able to confirm by using in vitro and in vivo models. These studies also demonstrated that the addition of VST does not improve the chemosensitizing effect of DAC alone.
Conclusions: Our data supports the strategy of epigenetic priming by employing DNMTI in RR-DLBCL patients in order to overcome resistance and improve their outcomes.
Keywords: Chemosensitization; Demethylating agents; Histone deacetylase inhibitors; Lymphoma; Phase Ib.
Figures

References
-
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–2045. doi: 10.1182/blood-2010-03-276246. - DOI - PMC - PubMed
-
- Elstrom RL, Martin P, Ostrow K, Barrientos J, Chadburn A, Furman R, et al. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clin Lymphoma Myeloma Leuk. 2010;10(3):192–196. doi: 10.3816/CLML.2010.n.030. - DOI - PubMed
-
- Cerchietti L, Leonard JP. Targeting the epigenome and other new strategies in diffuse large B-cell lymphoma: beyond R-CHOP. Hematology Am Soc Hematol Educ Program. 2013;2013:591–595. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

